If an antacid tablet weighed 1.6 grams, how many moles of gastric acid (HCl) would it neutralize? Use the results obtained in Data Tables 1 and 2 to explain and quantify your answer.
If an antacid tablet weighed 1.6 grams, how many moles of gastric acid (HCl) would it neutralize? Use the results obtained in Data Tables 1 and 2 to explain and quantify your answer.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.61QE
Related questions
Question
100%
If an antacid tablet weighed 1.6 grams, how many moles of gastric acid (HCl) would it neutralize? Use the results obtained in Data Tables 1 and 2 to explain and quantify your answer.
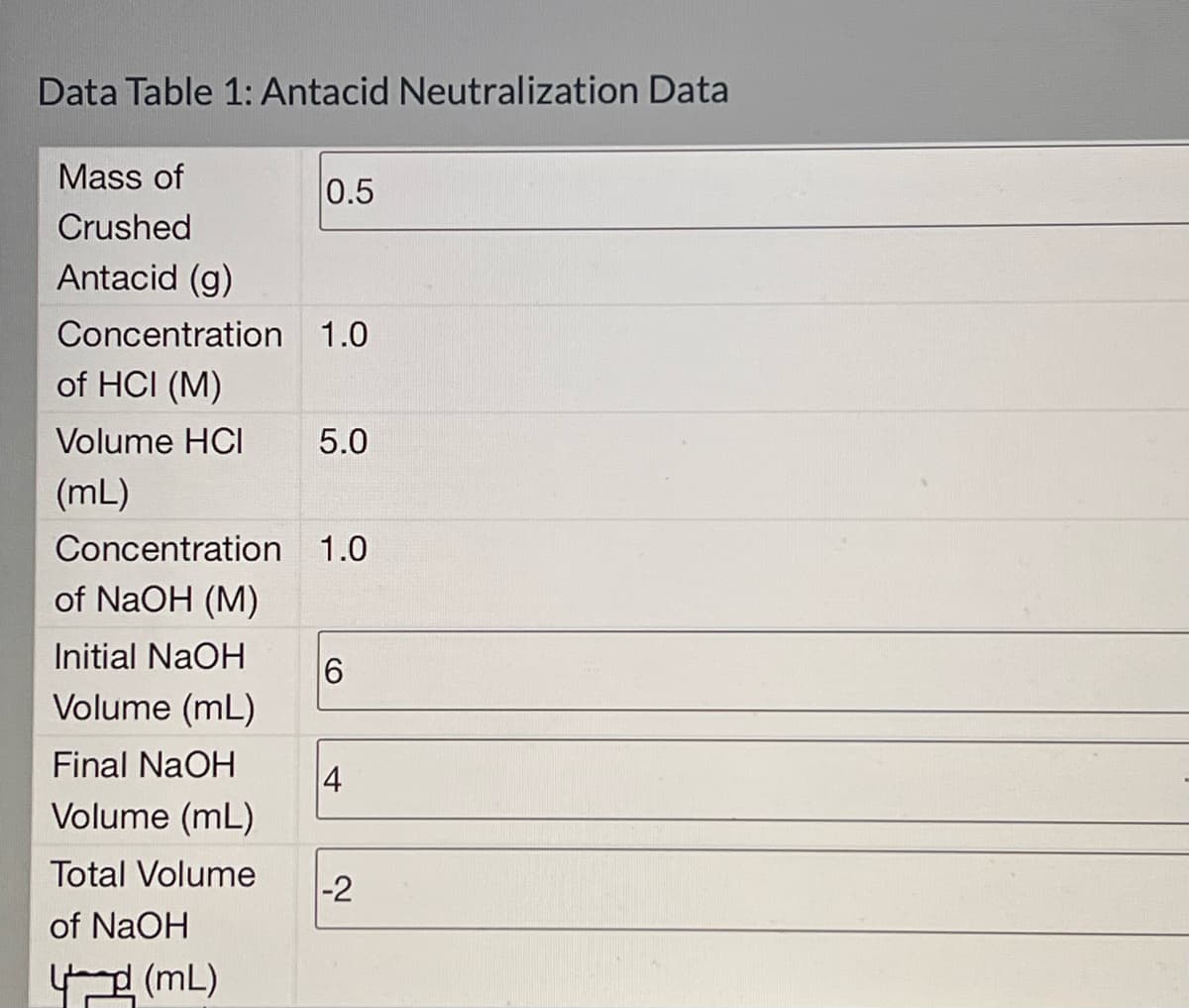
Transcribed Image Text:Data Table 1: Antacid Neutralization Data
Mass of
0.5
Crushed
Antacid (g)
Concentration 1.0
of HCI (M)
Volume HCI
5.0
(mL)
Concentration 1.0
of NaOH (M)
Initial NaOH
Volume (mL)
6
Final NaOH
4
Volume (mL)
Total Volume
-2
of NaOH
(mL)
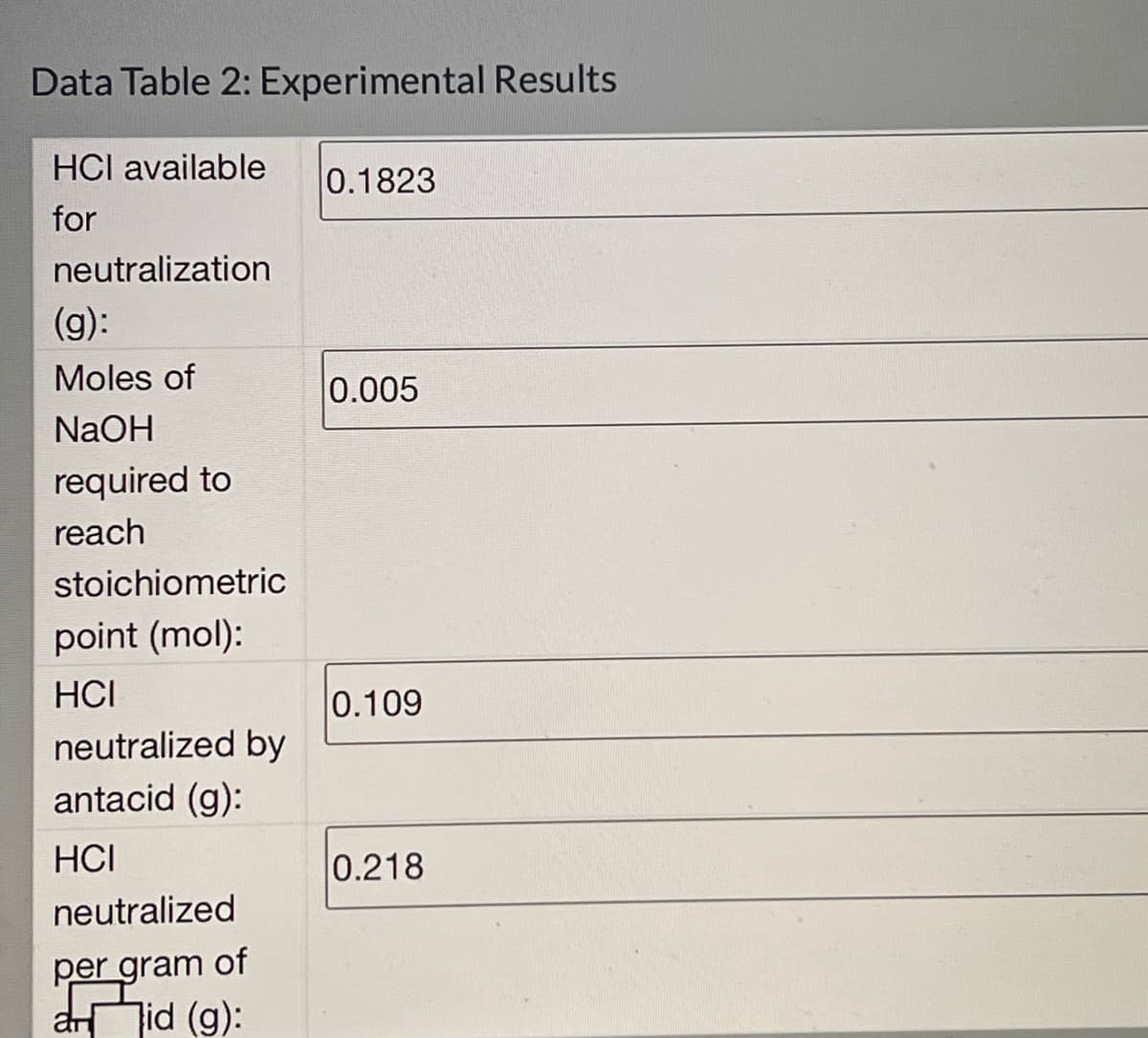
Transcribed Image Text:Data Table 2: Experimental Results
HCI available
0.1823
for
neutralization
(g):
Moles of
0.005
NaOH
reach
required to
stoichiometric
point (mol):
HCI
0.109
neutralized by
antacid (g):
HCI
0.218
neutralized
per gram of
aid (g):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning