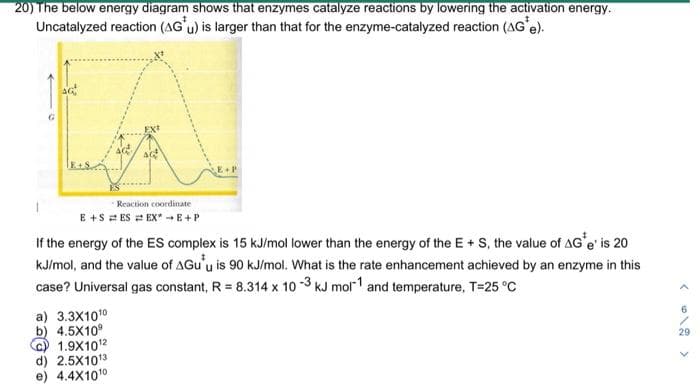
If the energy of the ES complex is 15 kJ/mol lower than the energy of the E+ S, the value of AG'e' is 20 kJ/mol, and the value of AGu'u is 90 kJ/mol. What is the rate enhancement achieved by an enzyme in this case? Universal gas constant, R = 8.314 x 10-3 kJ mol-1 and temperature, T-25 °C a) 3.3X1010 b) 4.5X109 1.9X10¹2 d) 2.5X10¹3 4.4X1010
Q: Draw Glycolysis. Please make sure to state all the enzymes and co-factors for each step of the…
A: Glycolysis is the metabolic pathway that converts 1 molecule of glucose to 2 molecules of pyruvate.…
Q: A technician prepares a buffer solution that will be used to facilitate the optimal pH for an enzyme…
A: A buffer is a solution with the ability to maintain the pH of a solution even if we add some…
Q: Describe how temperature affects the rate of an enzymatically catalyzed reaction.
A: All proteins are composed of monomers called amino acids. The amino acids form particular bonds with…
Q: Question 1 How many net ATP are made per glucose during glycolysis? O a.0 O b. 1 OC. 2 O d.5 O e. 8…
A: Glycolysis is a catabolic pathway and first step in cellular respiration in which a glucose molecule…
Q: D. Enumerate the ketone bodies. Describe the formation and fate of ketone bodies. Add a note on…
A: The liver converts fat into a trio of water-soluble molecules called ketone bodies when glucose is…
Q: The data to the right were collected for the myosin-catalyzed hydrolysis of ATP. Use these data to…
A: In order to solve this problem, first we need to use the same unit for each parameter of [ATP] and…
Q: Why is Vit.C important for our skin?
A: Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that plays a crucial role in…
Q: 3. Follow the two carbon atoms that enter the citric acid cycle (C₂). Are the two CO₂ molecules…
A: TCA cycle also called as citric acid cycle or tricarboxylic acid cycle. This is the second step in…
Q: Can you give me more expalination of Oxidation Deamination formation of -ketoglutaric acid and NH4+…
A: Oxidative deamination is a biochemical reaction that involves the removal of an amino group from an…
Q: The acute phase proteins. C-reactive protein.
A: When there is an inflammation, infection, and tissue damage in our body, a class of proteins called…
Q: Calculate the appropriate volume (in µL) of 3X loading buffer that should be added to 24.0 µL of a…
A: Dilution factor (DF) can be expressed both as the ratio of concentrations and as ratio of volumes.…
Q: What does the high-energy molecules adenosine triphosphate fforms and reduced forms of nicotinamide…
A: A high-energy molecule is adenosine triphosphate (ATP). It is created by cells and used as a source…
Q: Draw the catalytic mechanism of the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase…
A: Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is an enzyme that belongs to oxidoreductase class…
Q: Provide for each amino acid the names that will uniquely identify all ionisable groups and indicate…
A: The electrophoretic mobility of an amino acid is determined by its charge : mass ratio. The net…
Q: Soap, phospholipids and cholesterol are all amphipathic molecules. Provide a drawing of each and…
A: Amphipathic molecules are chemical compounds containing both polar and nonpolar portions in their…
Q: The solvent in this chromatography experiment is 75% butanol, 12% acetic acid, and 13% water. Is the…
A: Chromatography is a lab technique that is used for separating a mixture of molecules into individual…
Q: Analyze the reaction below and identify the major class of enzyme that catalyzes the the given…
A: Enzymes are biological catalysts that increases the rate of biochemical reactions. The enzymes can…
Q: 2. The overall reaction for the activation of a fatty acid to fatty acyl-CoA, with concomitant…
A: Fatty acid cannot enter mitochondria. To enter mitochondria, it must be activated by adding a CoA to…
Q: In this question, you need to discuss the results you obtained from the BSA standards to generate…
A: The BSA (Bovine Serum Albumin) assay is used to spectrophotometrically find the concentration of…
Q: Does the synthesis of fatty acids occur in the adipose tissue and liver? How?
A: Fatty acid synthesis is the process by which the body produces fatty acids from acetyl-CoA and…
Q: D. The correct sequence of cytochrome carriers in respiratory chain is (A) Cyt b-cyt c-cyt c1-cyt…
A: The respiratory chain is a series of protein complexes and electron carriers that facilitate the…
Q: An experiment was performed to determine the effects of an inhibitor on the breakdown of glycogen by…
A: In the enzyme assay conducted here, we are trying to figure out how the kinetics of the reaction is…
Q: THE SCIENTIFIC METHOD: DR. SEMMELWEIS’ CASE STUDY An example of the scientific method in action can…
A: The scientific method is a methodical strategy for problem-solving that is employed by scientists in…
Q: 2. Identify three metabolic pathways that pyruvate could directly take following glycolysis and be…
A: Glycolysis is the collection of 10 enzymatically catalysed reactions that oxidises a 1 molecule of…
Q: A set of biomolecules listed in the table at right are in solution at pH 6.8, when they are passed…
A: Ultrafiltration is a technique similar to dialysis where the sample containing a mixture of proteins…
Q: Describe the digestion and absorption of dietary lipids.
A: The digestion and absorption of dietary lipids is a complicated process that will be discussed in…
Q: The researchers did not study the effects of NADH, ADP and ATP on the enzyme. Given what you know of…
A: Enzyme are proteins molecules that catalyse the biochemical reactions. They are very crucial for…
Q: The increase of ATP is due to what pathway in the catabolism of glucose?
A: Cellular respiration is a collection of three metabolic pathways that generate ATP by the oxidation…
Q: Name the dipeptide using three-letter abbreviations.
A: A dipeptide is composed of 2 amino acid residues linked via a peptide bond. The amino acids can be…
Q: Give typed full explanation not a single word hand written otherwise leave it
A: The basic metabolic route known as the tricarboxylic acid (TCA) cycle, sometimes referred to as the…
Q: Sixty hours after aspirin ingestion, the patient’s blood pH has returned to normal (pH 7.4).…
A: Consider the reversible reaction shown below and it has an equilibrium. A ⇌ B All reactions strive…
Q: 1. Cytosine deamination, in which cytosine is mutated to uracil, occurs spontaneously in DNA at a…
A: Nitrogenous bases are cyclic compounds that contain nitrogen. They can be: Purines are heterocyclic…
Q: Synthesis signal. How does insulin stimulate glycogen synthesis?
A: Insulin is a peptide hormone that is synthesized in the beta cells of the Islets of Langerhans. The…
Q: The acidity of the stomach is maintained by the H*/K* ATPase in parietal cells of the gastric…
A: Hi! Thank you for the question. We are authorized to answer one question at a time, since you have…
Q: Which enzyme involved in the breakdown of fatty acids requires activated vitamin B₁2? O…
A: Vitamin B12 is a water soluble vitamin also known as cobalamin. Vitamin in necessary for the…
Q: 11.30) Identify the following as properties of amylose, amylopectin, both amylose and amylopectin,…
A: Polysaccharides are complex biomolecules composed of carbohydrates made up of long chains of…
Q: describe the role of mitochondria in initiating apoptosis. please describe and summarize in short…
A: Apoptosis, also known as programmed cell dress, is a natural process by which cells degrade their…
Q: How many Calories should there be in three grams of pure sucrose? C. 18 A. 4 B. 12 D. 8 E. M
A: Sucrose, the common sugar, is a disaccharide made up of glucose and fructose linked via a 1-1…
Q: Provide an example of an activity that would rely primarily on fats (lipids) for energy and another…
A: It's crucial to remember that throughout most activities, the body normally uses a combination of…
Q: Determine whether each of the inhibitors (or inhibitor characteristics) are involved in reversible…
A: Since you have posted a question with multiple sub parts, we will provide the solution only to the…
Q: Arachidonic acid, eicosapentaenoic acid, or dihomo-gamma-linolenic acid undergo reactions that…
A: The complete form of NSAIDs is Non-steroidal anti-inflammatory Drugs. These drugs are a class of…
Q: Proteins undergo  a process, called folding to establish their final, functional configuration.…
A: As per the central dogma of molecular biology, DNA contains the information needed to synthesize…
Q: How can heme be removed from the extracted DNA in Blood?
A: Heme is a molecule that contain iron. Numerous proteins, such as myoglobin and hemoglobin, depend on…
Q: At different glucose concentrations (2 mol of glucose, 4 mol of glucose, 8 mol of glucose), at what…
A: We know that glucose is the most favored respiratory substrate of our body. Glucose is efficiently…
Q: Fill in the missing identities and quantities of VIPs (atp/gtp, nash, qh, co2) to show the oxidation…
A: Glucose oxidation is the process of breaking down of glucose completely through the process…
Q: Patients suffering from Familial Hypercholesterolemia (FH) can carry a mutation in one of several…
A: LDL carries cholesteryl esters within it. LDL thereby transports cholesterol (in the form of…
Q: 1. Using a diagram or analogy, explain how energy is generated and used between plants and animals.…
A: The plant and the animal cells can be viewed as tiny energy factories that can facilitate the energy…
Q: Dehalogenase enzymes catalyze the clevage of C-X bonds. One such dehalogenase catalyzes the…
A: A dehalogenase enzyme removes a halogen atom from a substrate. It is found in certain bacteria such…
Q: 5.3 What is the key regulatory enzyme of glycolysis? How is this enzyme regulated In addition to…
A: Glycolysis is a metabolic mechanism in the cytoplasm of cells that converts glucose into two…
Q: Calculate the ΔG° for the making of 30 ATP from 30 ADP ATP + H2O ↔ ADP + Pi ∆G°’ = –30.5 kJ/mol…
A: If the change in standard Gibbs free energy (∆G0') of a forward reaction is 'x' , then the ∆G0' of…
Step by step
Solved in 3 steps

- Which of the following is true under the following conditions: an enzyme displaying Michaelis-Menten kinetics where the enzyme concentration is 10 nM, the substrate concentration is 45 mM, and the Km is 50 µM? a) The enzyme has low catalytic efficiency for the substrate. b)The rate of catalysis is near half-maximal velocity. c)The enzymatic reaction is near maximal velocity. d)Halving the substrate concentration has little effect on the catalytic rate. e) There is not enough information provided.Which of the following statements about a plot of V0 vs. [S] for an enzyme that follows Michaelis-Menten kinetics is false? a. As [S] increases, the initial velocity of reaction V0 also increases. b. At very high [S], the velocity curve becomes a horizontal line that intersects the y-axis at Km. c. Km is the [S] at which V0 = 1/2 Vmax. d. The shape of the curve is a hyperbola. e. The y-axis is a rate term with units of μm/min.In a Lineweaver-Burk graph, the lines representing the uninhibited and inhibited enzyme catalyzed reaction meet each other on the x-axis. The type of inhibition which is occurring is: a) competitive b) noncompetitive c) uncompetitive d) allosteric CO2 exerts direct activity upon hemoglobin by: a) blocking oxygen from binding to the heme group b) displacing BPG from the central cavity c) oxidizing Fe+2 to Fe+3 which does not bind oxygen d) forming an N-terminal carbamate which favors the T-state The dominant motif found in hemoglobin and myoglobin is: a) helix-turn-helix b) twisted beta sheet c) beta barrel d) random coil Which of these is an ketohexose? a) fructose b) glucose c) ribose d) erythrose Which of these is a constitutional isomer of d-glucose? a) fructose b) galactose c) l-glucose d) ribose Which of these is an enantiomer of d-glucose? a) d-fructose b) d- galactose c) l-glucose d) d-ribose Which of these is a diastereomer of…
- What general effects would you expect the following changes to have on the rate of an enzyme-catalyzed reaction for an enzyme that has its maximum activity at body temperature (about 37 °C/310.15 K)?(a) Lowering the reaction temperature from 313 K (40 °C)to 283 K (10 °C)(b) Adding a drop of a dilute HgCl2 solution(c) Adding an oxidizing agent, such as hydrogen peroxideWhich of the following best explains why enzyme catalysis is affected by a change in pH? A. Change in pH alters ionization states of serine in the active site involved in nucleophilic catalysis B. The ionization states of his, asp and glu involved in acid/base catalysis are altered with change in pH C. Change in pH alters ionization states of contact amino acids in the active site D. All enzymes have optimum pHYou begin to study enzyme Z, which catalyzes a simple reversible reaction that interconverts compound S and compound P. You observe that the ∆G´° for the S to P conversion to be –6 kJ/mol, and that compound S has ∆G´° for binding to enzyme Z of –15 kJ/mol, while compound P has a ∆G´° for binding to enzyme Z of –13 kJ/mol. Please explain the effect of enzyme Z on conversion of S to P. (Your answer should include a graph qualitatively showing energy versus reaction progress; however, you still need to explain youranswer in words!) not sure how to make the correct graph.
- Enzyme A catalyzes the reaction S → P and has a KM of 50 μM and a Vmax of 100 nM ∙ s−1. Enzyme B catalyzes the reaction S → Q and has a KM of 5 mM and a Vmax of 120 nM ∙ s−1. When 100 μM of S is added to a mixture containing equivalent amounts of enzymes A and B, after 1 minute which reaction product will be more abundant: P or Q?At what substrate concentration would an enzyme with a kcat of 25.0 s-1 and a KM of 3.5 mM operate at 25% of its maximal rate? How many reactions would the enzyme catalyze in 45 seconds when it is fully saturated with substate, assuming the enzyme has one active site?A particular reaction has a ΔG‡ of 30.0 kJ mol-1 at 25.0 °C. In the presence of an enzyme, the same reaction has a ΔG‡ of 1.50 kJ mol-1 at the same temperature. Calculate the rate enhancement of this enzyme. (R = 8.3145 J mol-1 K-1)
- You have been the only one who has been able to this. It has three other parts as well, A) Which Enzyme Catalyzes this reaction? choices are in image provided. B) What is ∆G°' for this reaction? Answer in Joules. K' = 19 C) If the concentration of Glucose-1-phosphate is 48.82 µM at equilibrium, what is the concentration of Glucose-6-phosphate in µM? D) If the reaction is not at equilibrium, what is ∆G' at 25°C if the concentration of Glucose-1-phosphate is 15.04µM and the concentration of Glucose-6-phosphate is 1.62 mM? Answer in Joules. Pay attention to units. Round to the correct number of significant figures. There are 103 µM in 1mM. Thank you and you are the winner for Genius of the day!!Assume you have an enzyme that catalyzes a reaction that breaks down dopachrome. At t = 0 s, the absorbance at 475 nm is 0.2 when you add the enzyme. At t = 30 s, would you expect the absorbance to be less than or greater than 0.2?Given the following information, calculate the catalytic efficiency of the enzyme. Step by step please [S] = 100 mM k1 = 10 sec-1 k2 = 3000 sec-1 k-1 = 20 sec-1 [E]T = 1 \muμM

