If the Favorskii rearrangement of 2-chlorocyclohexanone is carried out using sodium ethoxide in ethanol, the product is ethyl cyclopentanecarboxylate. -OEt .CI EtO-Na+ ELOH Propose a mechanism for this reaction.
If the Favorskii rearrangement of 2-chlorocyclohexanone is carried out using sodium ethoxide in ethanol, the product is ethyl cyclopentanecarboxylate. -OEt .CI EtO-Na+ ELOH Propose a mechanism for this reaction.
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
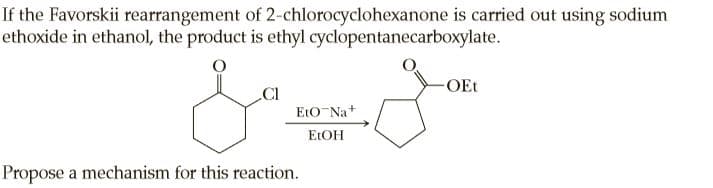
Transcribed Image Text:If the Favorskii rearrangement of 2-chlorocyclohexanone is carried out using sodium
ethoxide in ethanol, the product is ethyl cyclopentanecarboxylate.
-OEt
.CI
EtO-Na+
ELOH
Propose a mechanism for this reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT