OH 1. Hg(OAc)2, H2O 2. NABH4 CH3 .CH3 H2C° H3C d-catalyzed addition of water to an alkene yields an alcohol with Markovnikov regiochemistry. The electrophilic H¯ adds to the sp² carbon with the most hydrogens to yield the most stab ocation intermediate, which then adds water to give the product alcohol. Because a carbocation intermediate is formed, rearrangements can occur prior to the addition of water. void the possibility of rearrangement and still give a Markovnikov alcohol, alkenes can instead be treated with mercury(II) acetate in aqueous THF and then subsequently reduced with se phydride. This reaction proceeds through a cyclic mercurinium ion intermediate which cannot rearrange. Water adds to the cyclic intermediate at the most substituted carbon to give an anomercury alcohol. The reduction step with sodium borohydride is complex and involves radicals. w curved arrows to show the movement of electrons in this step of the mechanism. row-pushing Instructions AcO-Hg-OAc AcO-Hg OAC
OH 1. Hg(OAc)2, H2O 2. NABH4 CH3 .CH3 H2C° H3C d-catalyzed addition of water to an alkene yields an alcohol with Markovnikov regiochemistry. The electrophilic H¯ adds to the sp² carbon with the most hydrogens to yield the most stab ocation intermediate, which then adds water to give the product alcohol. Because a carbocation intermediate is formed, rearrangements can occur prior to the addition of water. void the possibility of rearrangement and still give a Markovnikov alcohol, alkenes can instead be treated with mercury(II) acetate in aqueous THF and then subsequently reduced with se phydride. This reaction proceeds through a cyclic mercurinium ion intermediate which cannot rearrange. Water adds to the cyclic intermediate at the most substituted carbon to give an anomercury alcohol. The reduction step with sodium borohydride is complex and involves radicals. w curved arrows to show the movement of electrons in this step of the mechanism. row-pushing Instructions AcO-Hg-OAc AcO-Hg OAC
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter10: Alcohols
Section: Chapter Questions
Problem 10.52P: Alcohols are important for organic synthesis, especially in situations involving alkenes. The...
Related questions
Question
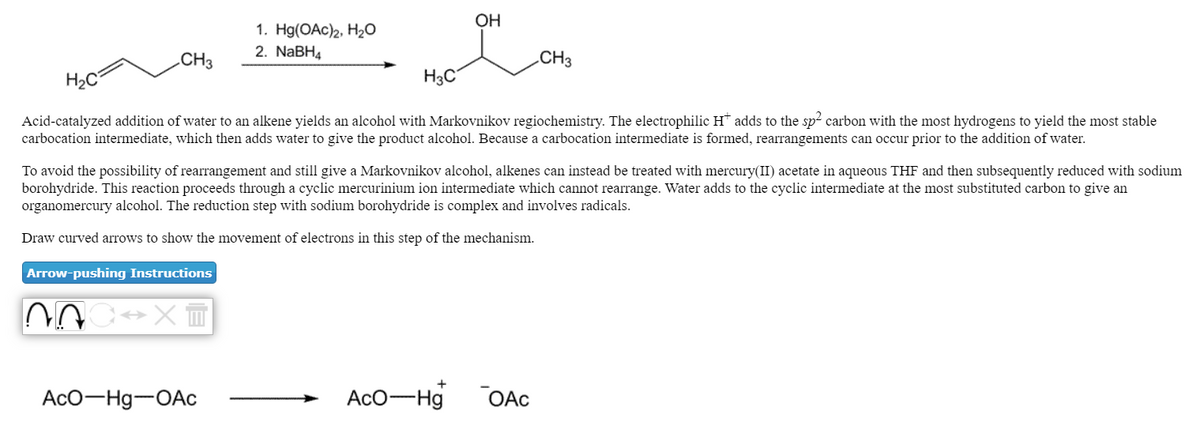
Transcribed Image Text:OH
1. Hg(OAc)2, H20
2. NaBH4
.CH3
CH3
H2C
H3C
Acid-catalyzed addition of water to an alkene yields an alcohol with Markovnikov regiochemistry. The electrophilic H* adds to the sp2 carbon with the most hydrogens to yield the most stable
carbocation intermediate, which then adds water to give the product alcohol. Because a carbocation intermediate is formed, rearrangements can occur prior to the addition of water.
To avoid the possibility of rearrangement and still give a Markovnikov alcohol, alkenes can instead be treated with mercury(II) acetate in aqueous THF and then subsequently reduced with sodium
borohydride. This reaction proceeds through a cyclic mercurinium ion intermediate which cannot rearrange. Water adds to the cyclic intermediate at the most substituted carbon to give an
organomercury alcohol. The reduction step with sodium borohydride is complex and involves radicals.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
AcO-Hg-OAc
AcO-Hg
OAc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
