If you have 155 mL solution of a 0.762 M FeCb solution, how many g FeCb are contained in this sample? 155 ml solution motFect x 0.762 STARTING AMOUNT molFeC. 155 ml solution 0.762 mol FeCh x- 1 mol FeCl ADD FACTOR DELETE ANSWER RESET *( ) 1000 0.1 124 4.92 1.92 0.203 162.2 55.85 12.6 35.45 100 25.1 7.28 x 10 6.60 1 0.762 155 0.118 19.2 0.01 mol FeCt g FeCl/mol FeCl L solution g FeCh g CF M FeCb g Fe* ml solution mol FeCl:/L solution
If you have 155 mL solution of a 0.762 M FeCb solution, how many g FeCb are contained in this sample? 155 ml solution motFect x 0.762 STARTING AMOUNT molFeC. 155 ml solution 0.762 mol FeCh x- 1 mol FeCl ADD FACTOR DELETE ANSWER RESET *( ) 1000 0.1 124 4.92 1.92 0.203 162.2 55.85 12.6 35.45 100 25.1 7.28 x 10 6.60 1 0.762 155 0.118 19.2 0.01 mol FeCt g FeCl/mol FeCl L solution g FeCh g CF M FeCb g Fe* ml solution mol FeCl:/L solution
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 10P
Related questions
Question
100%
How do I fill this out?
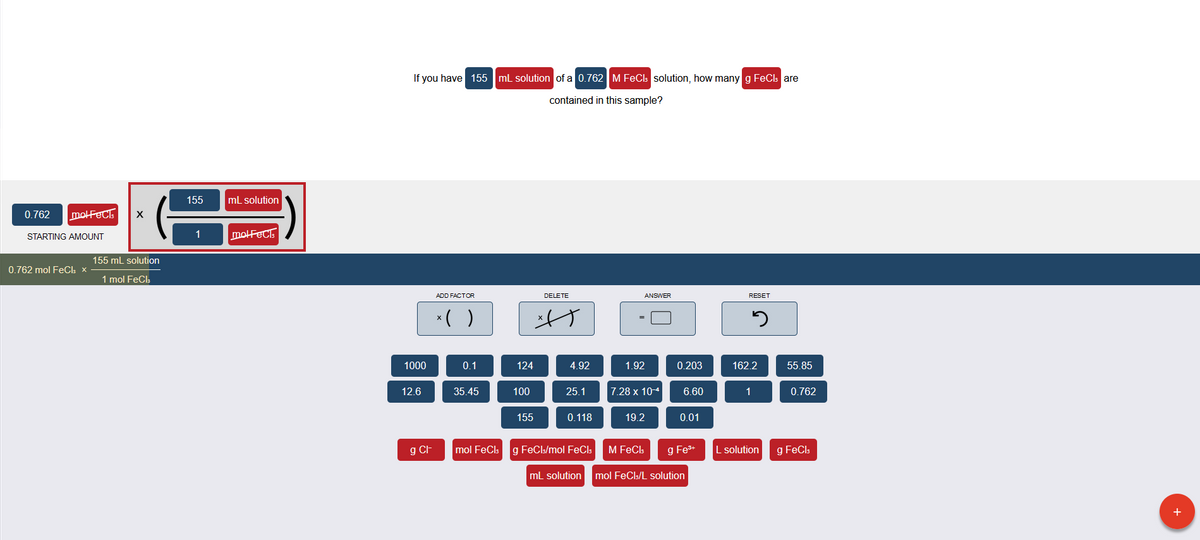
Transcribed Image Text:If you have 155 mL solution of a 0.762 M FeCls solution, how many g FeCls are
contained in this sample?
155
mL solution
0.762
molFeCls
STARTING AMOUNT
1
molFeCE
155 mL solution
0.762 mol FeC: ×
1 mol FeCl:
ADD FACTOR
DELETE
ANSWER
RESET
*( )
1000
0.1
124
4.92
1.92
0.203
162.2
55.85
12.6
35.45
100
25.1
7.28 x 10-4
6.60
0.762
155
0.118
19.2
0.01
g CH
mol FeCls
g FeCls/mol FeCIs
M FeCls
g Fe+
L solution
g FeCls
mL solution
mol FeCls/L solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning