Ignition wires heat Thermometer Stirrer sample A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods In an experiment, a 1.0580 g sample of B-D-fructose (C6H12O6) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.284x103 g of water. During the combustion the temperature increases from 26.32 to 29.03 °C. The heat capacity of water is 4.184 J g °C. Water The heat capacity of the calorimeter was determined in a previous experiment to be 991.6 J/oC Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of B-D-fructose based on these data 6 H2O(I)6 CO2(g) Energy CH12O6(s)6O2(g) Insulated Sample dish Burning sample Steel bomb kJ/mol Molar Heat of Combustion outside chamber Combustion (bomb) calorimeter.
Ignition wires heat Thermometer Stirrer sample A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods In an experiment, a 1.0580 g sample of B-D-fructose (C6H12O6) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.284x103 g of water. During the combustion the temperature increases from 26.32 to 29.03 °C. The heat capacity of water is 4.184 J g °C. Water The heat capacity of the calorimeter was determined in a previous experiment to be 991.6 J/oC Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of B-D-fructose based on these data 6 H2O(I)6 CO2(g) Energy CH12O6(s)6O2(g) Insulated Sample dish Burning sample Steel bomb kJ/mol Molar Heat of Combustion outside chamber Combustion (bomb) calorimeter.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 112AE: In a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each...
Related questions
Question
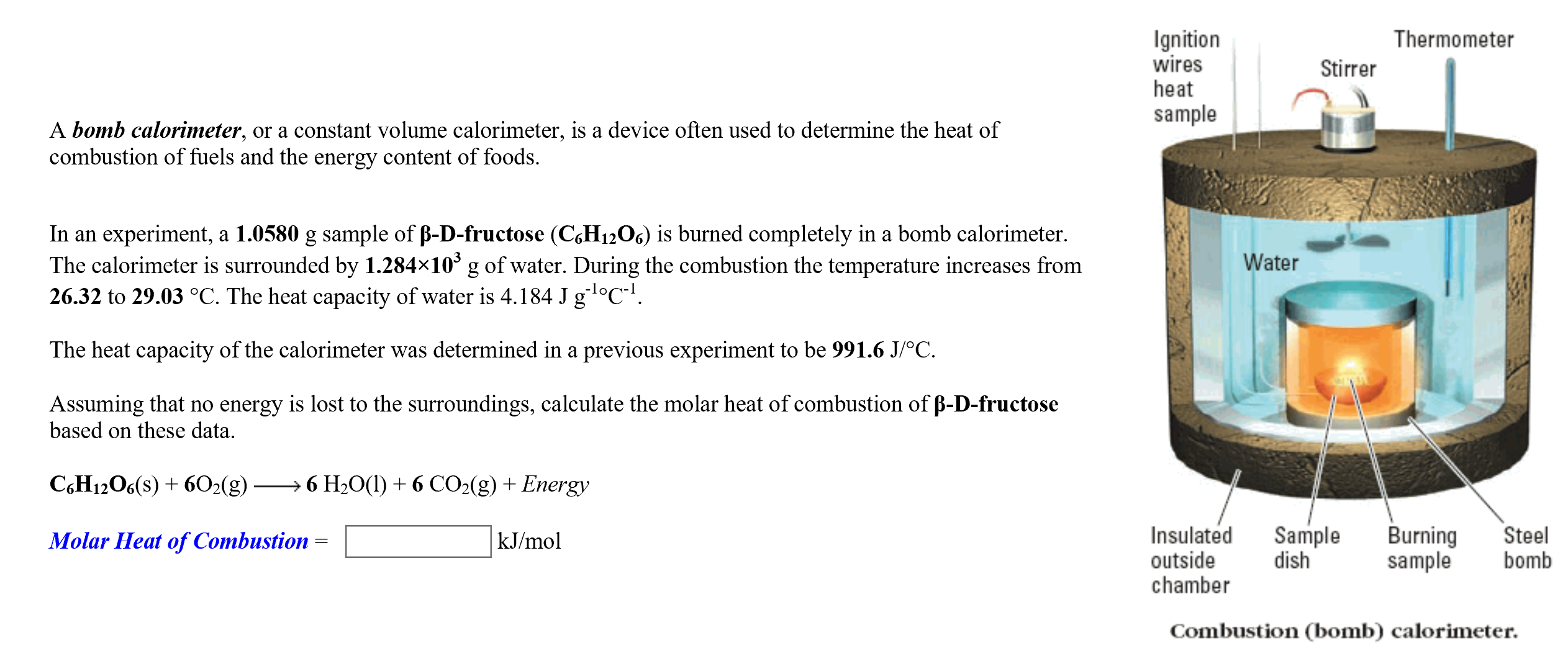
Transcribed Image Text:Ignition
wires
heat
Thermometer
Stirrer
sample
A bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of
combustion of fuels and the energy content of foods
In an experiment, a 1.0580 g sample of B-D-fructose (C6H12O6) is burned completely in a bomb calorimeter.
The calorimeter is surrounded by 1.284x103 g of water. During the combustion the temperature increases from
26.32 to 29.03 °C. The heat capacity of water is 4.184 J g °C.
Water
The heat capacity of the calorimeter was determined in a previous experiment to be 991.6 J/oC
Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of B-D-fructose
based on these data
6 H2O(I)6 CO2(g) Energy
CH12O6(s)6O2(g)
Insulated
Sample
dish
Burning
sample
Steel
bomb
kJ/mol
Molar Heat of Combustion
outside
chamber
Combustion (bomb) calorimeter.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning