In a constant-pressure calorimeter, 55.0 mL of 0.350 M Ba(OH), was added to 55.0 mL of 0.700 M HCI. The reaction caused the temperature of the solution to rise from 23.03 °C to 27.80 °C. If the solution has the same density and specific heat as water (1.00 g/mL and 4.184J/g °C,) respectively), what is AH for this reaction (per mole H₂O produced)? Assume that the total volume is the sum of the individual volumes. AH= x10 TOOLS kJ/mol H₂O
In a constant-pressure calorimeter, 55.0 mL of 0.350 M Ba(OH), was added to 55.0 mL of 0.700 M HCI. The reaction caused the temperature of the solution to rise from 23.03 °C to 27.80 °C. If the solution has the same density and specific heat as water (1.00 g/mL and 4.184J/g °C,) respectively), what is AH for this reaction (per mole H₂O produced)? Assume that the total volume is the sum of the individual volumes. AH= x10 TOOLS kJ/mol H₂O
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 127CWP: In a coffee-cup calorimeter, 150.0 mL of 0.50 M HCI is added to 50.0 mL of 1.00 M NaOH to make 200.0...
Related questions
Question
Payalben
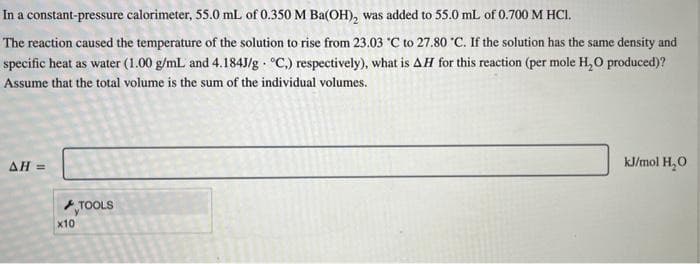
Transcribed Image Text:In a constant-pressure calorimeter, 55.0 mL of 0.350 M Ba(OH), was added to 55.0 mL of 0.700 M HCI.
The reaction caused the temperature of the solution to rise from 23.03 °C to 27.80 °C. If the solution has the same density and
specific heat as water (1.00 g/mL and 4.184J/g °C,) respectively), what is AH for this reaction (per mole H₂O produced)?
Assume that the total volume is the sum of the individual volumes.
AH=
x10
TOOLS
kJ/mol H₂O
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax