In a lab project, a student obtained a colored basic solution whose concentration has to be determined. The student wants to perform a titration but he does not know which indicator he can use. Can he perform a titration without an indicator? Which method can he use to determine the concentration and why?
In a lab project, a student obtained a colored basic solution whose concentration has to be
determined. The student wants to perform a titration but he does not know which indicator he
can use. Can he perform a titration without an indicator? Which method can he use to
determine the concentration and why?
Yes he can perform the titration without an indicator as well.
Measuring the pH of the solution after every small amount of acid added and plotting the curve between pH and volume of acid will look something like as shown below.
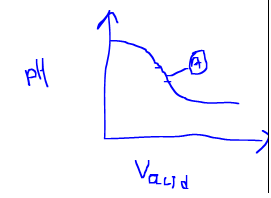
Hence the point A is representing the equivalence point of the titration i.e the the middle point where we see a sharp change in the pH of the solution by a small addition of the acid
Hence the volume of acid required for the titration is now known
Hence the concentration of unknown can be calculated
Step by step
Solved in 3 steps with 1 images









