In chemical reactions, heat is converted into chemical energy (the potential energy stored in chemical bonds) or vice versa. Bond energy is the energy required to break one mole of the bond in the gas phase. Since it takes energy to break a bond, bond energies are always positive. Conversely, energy is released when bonds are formed. Thus, the enthalpy change for a reaction can be approximated from Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HẢ ? ΔΗ Σ(ΔΗ breaking) + Σ(ΔΗ forming) Value Units where H represents bond energies for the breaking (positive bond energy) or forming (negative bond energy) of a bond and Hrxn represents the overall enthalpy for the AHo, = reaction. Submit Use the table to answer questions about bond energies. Bond energy Bond (kJ/mol ) Part C C-C 347 Calculate the bond energy per mole for forming all the bonds of water molecules, H2O. C=C 611 СН Express your answer to three significant figures and include the appropriate units. 414 C-O • View Available Hint(s) 360 C=OinCO2 799 0-O HẢ 142 0=0 498 Value Units ΔΗΗ,0- 464 Submit Part D Calculate the bond energy per mole for forming all the bonds of carbon dioxide, CO2. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HẢ Value Units ДНсо, - Submit
In chemical reactions, heat is converted into chemical energy (the potential energy stored in chemical bonds) or vice versa. Bond energy is the energy required to break one mole of the bond in the gas phase. Since it takes energy to break a bond, bond energies are always positive. Conversely, energy is released when bonds are formed. Thus, the enthalpy change for a reaction can be approximated from Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HẢ ? ΔΗ Σ(ΔΗ breaking) + Σ(ΔΗ forming) Value Units where H represents bond energies for the breaking (positive bond energy) or forming (negative bond energy) of a bond and Hrxn represents the overall enthalpy for the AHo, = reaction. Submit Use the table to answer questions about bond energies. Bond energy Bond (kJ/mol ) Part C C-C 347 Calculate the bond energy per mole for forming all the bonds of water molecules, H2O. C=C 611 СН Express your answer to three significant figures and include the appropriate units. 414 C-O • View Available Hint(s) 360 C=OinCO2 799 0-O HẢ 142 0=0 498 Value Units ΔΗΗ,0- 464 Submit Part D Calculate the bond energy per mole for forming all the bonds of carbon dioxide, CO2. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HẢ Value Units ДНсо, - Submit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 79QAP: Which statement(s) is/are true about bond enthalpy? (a) The bond energy for a triple bond between A...
Related questions
Question
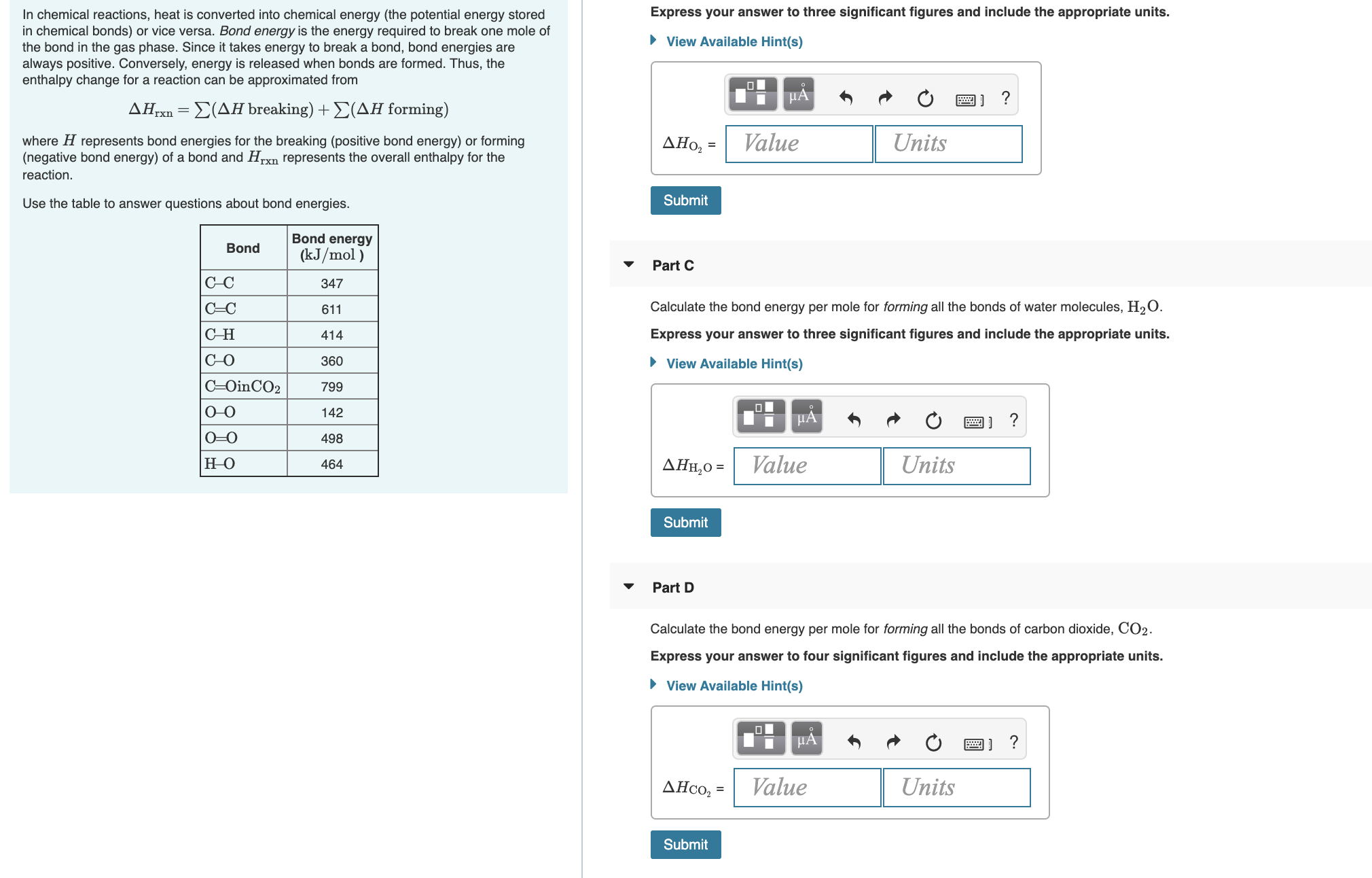
Transcribed Image Text:In chemical reactions, heat is converted into chemical energy (the potential energy stored
in chemical bonds) or vice versa. Bond energy is the energy required to break one mole of
the bond in the gas phase. Since it takes energy to break a bond, bond energies are
always positive. Conversely, energy is released when bonds are formed. Thus, the
enthalpy change for a reaction can be approximated from
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
HẢ
?
ΔΗ
Σ(ΔΗ breaking) + Σ(ΔΗ forming)
Value
Units
where H represents bond energies for the breaking (positive bond energy) or forming
(negative bond energy) of a bond and Hrxn represents the overall enthalpy for the
AHo, =
reaction.
Submit
Use the table to answer questions about bond energies.
Bond energy
Bond
(kJ/mol )
Part C
C-C
347
Calculate the bond energy per mole for forming all the bonds of water molecules, H2O.
C=C
611
СН
Express your answer to three significant figures and include the appropriate units.
414
C-O
• View Available Hint(s)
360
C=OinCO2
799
0-O
HẢ
142
0=0
498
Value
Units
ΔΗΗ,0-
464
Submit
Part D
Calculate the bond energy per mole for forming all the bonds of carbon dioxide, CO2.
Express your answer to four significant figures and include the appropriate units.
• View Available Hint(s)
HẢ
Value
Units
ДНсо, -
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning