In each reaction box, place the best reagent and conditions from the list below. Br 1) Br 2) 3) 4) Br2, H20 PBr3 ВныTHF CH;CO0- (CH3);CO dilute H30* NABH4 H2CrO4 H2O2, NAOH, H2o
In each reaction box, place the best reagent and conditions from the list below. Br 1) Br 2) 3) 4) Br2, H20 PBr3 ВныTHF CH;CO0- (CH3);CO dilute H30* NABH4 H2CrO4 H2O2, NAOH, H2o
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 18E
Related questions
Question
100%
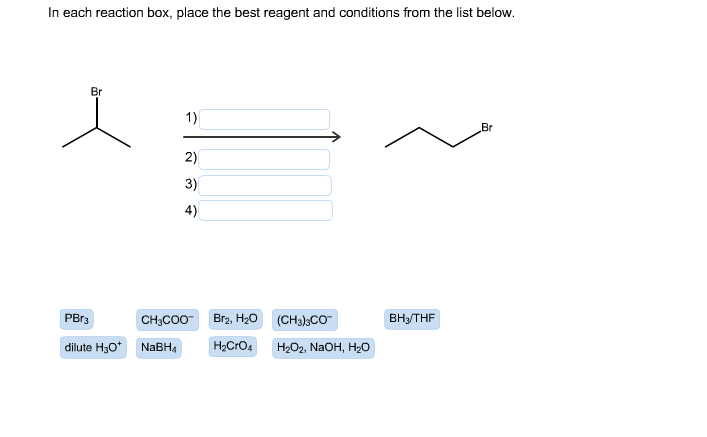
Transcribed Image Text:In each reaction box, place the best reagent and conditions from the list below.
Br
1)
Br
2)
3)
4)
Br2, H20
PBr3
ВныTHF
CH;CO0-
(CH3);CO
dilute H30* NABH4
H2CrO4
H2O2, NAOH, H2o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
