In ending the titration of KHP, you leave a half-drop of the NaOH hanging from the tip of the buret. The rest of the experiment is done flawlessly. The calculated % acetic acid in the vinegar will be correct wrong, but can be either too high or too low slightly low slightly high A one drop error (0.05 ml) was made in measuring the volume of NaOH needed to titrate the KHP. If 16.00 ml was actually used, the error will cause what percen error in the calculated molarity of NaOH Greater than 1.0% O Less than 0.25% Between 0.25 and 0.5 % Between 0.5 % and 1.0%
In ending the titration of KHP, you leave a half-drop of the NaOH hanging from the tip of the buret. The rest of the experiment is done flawlessly. The calculated % acetic acid in the vinegar will be correct wrong, but can be either too high or too low slightly low slightly high A one drop error (0.05 ml) was made in measuring the volume of NaOH needed to titrate the KHP. If 16.00 ml was actually used, the error will cause what percen error in the calculated molarity of NaOH Greater than 1.0% O Less than 0.25% Between 0.25 and 0.5 % Between 0.5 % and 1.0%
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
Please explain
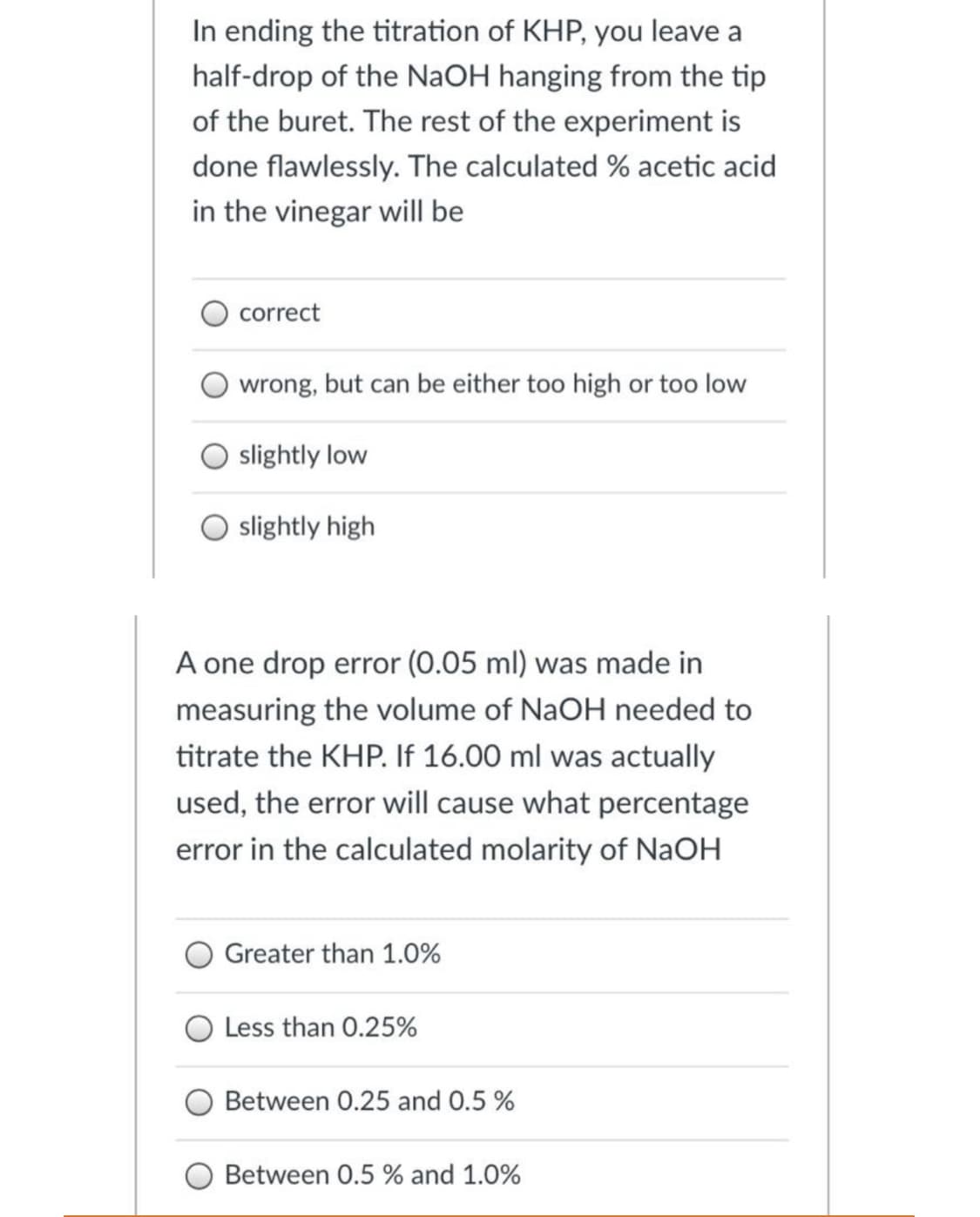
Transcribed Image Text:In ending the titration of KHP, you leave a
half-drop of the NaOH hanging from the tip
of the buret. The rest of the experiment is
done flawlessly. The calculated % acetic acid
in the vinegar will be
correct
wrong, but can be either too high or too low
slightly low
slightly high
A one drop error (0.05 ml) was made in
measuring the volume of NaOH needed to
titrate the KHP. If 16.00 ml was actually
used, the error will cause what percenta
error in the calculated molarity of NaOH
Greater than 1.0%
Less than 0.25%
Between 0.25 and 0.5 %
Between 0.5 % and 1.0%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you



