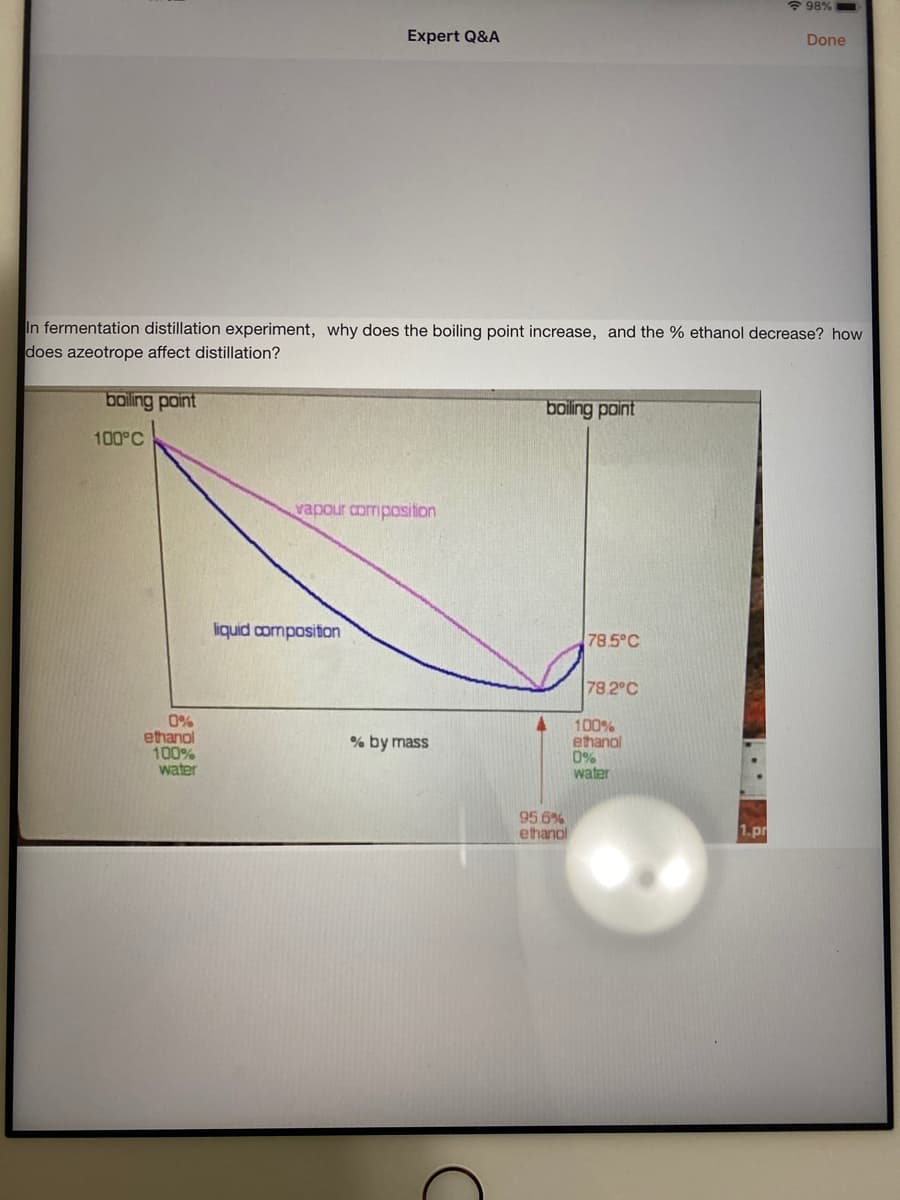
In fermentation distillation experiment, why does the boiling point increase, and the % ethanol decrease? how does azeotrope affect distillation? boiling point boling point 100°C vapour composition liquid compositon 78.5°C 78.2°C 0% ethanol 100% water 100% ethanol 0% water % by mass 95 6% ethanol 1.pr
Q: Iron(II) chloride (melting point 677 °C) and potassium chloride (melting point 776 °C) form the…
A:
Q: When performing a recrystallization of an impure solid, why is it important to allow the heated…
A: Recrystallisation is one of the most used technique in chemistry to obtain the pure compound from an…
Q: A liquid mixture consists of 3.0 mol of compound A, with melting point of 144 K, and 2.0 mol of…
A:
Q: A 10-in diameter opening in a carboy filed with a methanol and an aldehyde, 0.2 mol fraction…
A: Given as Diameter of opening=10 in ymethanol=0.2 T=40 oC Psat=125+100 χ mmHg Molecular weight of…
Q: Describe a binary eutectic diagram and show all phase regions with all the relevant information for…
A: The binary eutectic phase diagram explains the chemical behavior of two immiscible (unmixable)…
Q: Sketch the non-ideal vapour pressure-composition curve of element X and nitrous acid, HNO2 where the…
A: The graphical representation of the non-ideal solution in which vapor pressure is on y-axis and the…
Q: Sketch the phase diagram of the system NH3/N2H4 given that the two substances do not form a compound…
A: The phase diagram displays the physical states of a material at varying temperature and pressure…
Q: Sketch the solid-liquid phase diagram of 2,6-xylenol and tert-butanol. Pure tert-butanol has a…
A: For the purpose of convenience, tert-butanol has been considered as liquid A and 2,6-xylenol has…
Q: Consider the following two white solids below and their physical properties based on information…
A: From the given information we get the answer
Q: boiling point boiling point BPtof pure A vapour composition liquid composition Bpt of pure B O A 1.0…
A: Given data :-
Q: boiling point boiling point BPtof pure A vapour composition liquid composition Bpt of pure B OA 1.0B…
A:
Q: Question attached
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you.…
Q: Co-Perciptation
A: In this question we have to explain the following types of Co-Perciptation 1- Surface Adorption 2…
Q: Consider the given liquid-vapor diagram: 375 370 365 360 355 350 345 340 0.1 02 0.8 03 04 05 0.5 0.7…
A: The given liquid-liquid phase diagram states the composition of the vapor and corresponds to the…
Q: b) This sample is heated to T = 110 °C. Give the mass of the phases present at this temperature and…
A: In an ideal mixture of two liquids, both the liquids have different in boiling point. Therefore,…
Q: 1. At 365 K, the vapor pressures of benzene and methylbenzene are 142 and 57 kPa, respectively.…
A: In order to solve this question, we apply raoult's law for ideal solutions and the vapor pressure of…
Q: Need solution to all parts urgently The solubility of 1,1,2-trichloroethane in water is 4500…
A: In this question , we will use Henry's law equation which is P( partial pressure of gas) =…
Q: TIK |210 Compositon of one phase P.1 Vapour 292 290 P+2 Composton fsecond phase P2 Liquid 273 P.1…
A:
Q: Uranium tetrafluoride and zirconium tetrafluoride melt at 1035 °C and 912 °C respectively. They form…
A: The melting points of uranium tetrafluoride and zirconium tetrafluoride are given as 1035°C and…
Q: What are the reasons why there are minimum and maximum azeotrope? How does this relate to the…
A: Minimum azeotrope is a solution whose boiling point is lower than the boiling point of the boiling…
Q: stion 11 Based on the graph, what is the estimated number of theoretical plates of the fractional…
A:
Q: 7.The diagram below is an example of Undistillable Mixtures (T vs Mole Fraction).How many degrees of…
A: Degree of freedom for the given system is given as F = C- P+1 F is degree of freedom C is no. Of…
Q: What are azeotropes, and how do they affect a distillation process? Azeotropic distillation is a…
A: In order to separate mixtures in a boiling liquid mixture, an approach called azeotropic…
Q: Basis for recrystallization Nernst Distribution Law differences in solubilities of a solid and an…
A:
Q: The following temperature/composition data were obtained for a mixture of two liquids A and B at…
A: Part-a).Please find the plot below.
Q: Phase Diagram for Solution Critical Point 217.75 (Critical pressure) Normal freening point 1.00 New…
A: normal boiling point is the temperature at which the vapour pressure is equal to 1 atm or 760 mmHg.…
Q: 3. Draw (illustrate) a hypothetical phase diagram incorporating the following salient features: A.…
A: Since you have posted multiple questions, we have answered the first one for you. To get the…
Q: An azeotropic mixture can be separated by fractional For azeotropic mixtures, the liquid and vapour…
A: Azeotropic mixtures are the ones that have same composition in liquid as well as in vapour phase and…
Q: atch the following: Freezing point depression The effect of supercooling Freezing point of pure…
A: Let us discuss the phase diagram first and then find out the respective points.
Q: The solvent for an organic reaction is prepared by mixing 70.0 mL of acetone (C3H6OC3H6O) with 56.0…
A: Vapor pressure of the mixture is calculated using the Roulte's law, solvent's partial vapor pressure…
Q: TRUE OR FALSE: 1. Solids such as naphthalene when heated will pass thru a process called…
A: 1. Solids such as naphthalene when heated will pass thru a process called condensation prior to…
Q: Henry's law constant for CO2 in water is 0.034 mol/kg bar. How many moles CO2 will dissolve in 500g…
A:
Q: 1 mol of gas containing O₂ 20%, N₂ 78%, and SO2 2%, find the composition of the gas on an SO₂ - free…
A: Given, 1 mol of gas contains : Sl.no Component Percentage composition 1 O2 20% 2 N2 78% 3…
Q: Consider the following two white solids below and their physical properties based on information…
A: We are given a mixture of camphor and cinnamic acid to separate.
Q: In an experiment to determine the relative molecular mass of ethanoic acid in an organic solvent,…
A: The molecular mass of ethanoic acid is determined at different temperatures. The mass of the…
Q: The partial molar volumes of water and ethanol in a solution with XH20 = 0.45 at 25°C are 17.0 and…
A: Given partial molar volume of water, VmW = 17.0 cm3/mol or 17.0 cm3.mol-1 partial molar volume of…
Q: atredoWith reference to the liquid-solid phase diagram below, label the four regions of the diagram…
A: The existence of equilibrium between different phases that is solid, liquid, and vapor has been…
Q: A student obtained a solid product in laboratory synthesis. To verify the identity of the solid, she…
A: By merely looking at any compound we cannot identify what its structure is. Melting point is an…
Q: Given the following mixture of two compounds 50.00 mL of X (MW =78.00 g/mol)(density 1.219 g/mL) and…
A:
Q: Use Raoult's law to calculate vapour liquid quilibrium behaviour for a multicomponent mixture of…
A:
Q: A liquefied mixture of n-butane, n-pentane, and n-hexane has the following composi- tion in percent:…
A: The fraction of a particular entity in a mixture containing that entity amongst other chemical…
Q: What is the molar ratio of 1,1-dichloroethane (11DCE) to 1,2-dichloroethane (12DCE) in the gas phase…
A: Let the mole fraction of 11DCE and 12DCE in the solution be X1 and X2 respectively. The value of X1…
Q: What is the molar ratio of 1,1-dichloroethane (11DCE) to 1,2-dichloroethane (12DCE) in the gas phase…
A: Rault’s law narrates that “the pressure of a provided gas in a specific mixture is obtained by…
Q: What mass (in kg) of ethylene glycol would be needed to add to 5.0 kg of water to raise the boiling…
A: given , mass of water = 5.0 Kg raise the boiling point of water by 2.0 oC Kbp H2O = +0.5121 oC/m…
Q: A liquid mixture of 50% mole n-butane and 50% mole n-pentane at 60oC and brought to a total pressure…
A: Solution -
Q: Q3: An equimolar liquid mixture of ethanol and water is in equilibrium with its vapor at 50° C.…
A: Given: Temperature of vapor = 50 C = (50 + 273) = 323 K For water, A = 18.30, B = 3816.44, C =…
Q: Jeneen v A certain liquid X has a normal bolling polnt of 120.70 °C and a bolling potnt elevation…
A: Given : Normal boiling point of liquid X = 120.70 oC Mass of solvent i.e liquid X = 800 g = 0.800 Kg…
Step by step
Solved in 2 steps

- The best liquid mixtures for separation by simple distillation need to have boiling points that differ by at least ___________ oC. 10 20 30 404.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint. The datd of four trials was collected. Please answer the following questions 4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr experiment 5) calculate the percent error in you value of ksp using reference that is found as the theoretical value. 6) why was the temperature of the saturated solution recorded?4.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint. The datd of four trials was collected. Please answer the following questions 1) calculate the total volume and moles of NaOH required to reach the endpoint for each trial. 2) calculate the molar solubility of potassium hydrogen tartate ( in mol/L) for each trial. 3) calculate the average molar solubility of potassium hydrogen tartate for the four trials. What is the average solubility of potassium hydrogen tartrate in g/L? 4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr…
- Solid Na2S is slowly added to a solution of 1.0 x 10-3 M Cr3+ (with no change in volume). At what [S2-] will Cr2S3 start to ppt? For Cr2S3, Ksp = 1.0 x 10-20Hexanoic acid was added to an immiscible biphasic solvent system, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D2) of hexanoic acid in water with respect to CCl4.Hexanoic acid was added to an immiscible biphasic solvent sysem, water and CCl4 at 20.0OC and the equilibrium concentrations of hexanoic acid were determined to be 3.66 g/L in H2O and 67.0 g/L in CCl4. Caluclate the distrubution coeffiecent (D1) of hexanoic acid in CCl4 with respect to water.
- A 18 g of unknown organic sample was dissolve in 756 mL of benzene. The boiling point of benzene was increased by 3.36oC. As the first step of analysis, determine the moecular weight of the unknow sample? Kb of benzene= 2.64oC/m Bb of benzene = 80.09 oC density of benzene = 0.874 g/mL at 25 °C Answer in whole number, no units required.Using the phase diagram for mixtures of cyclohexane and toluene, estimate: A) Boiling point of pure toluene _________ B) Boiling point (Tbp) of solution with molar fraction of toluene 30%.______ C) Molar fraction of toluene (in %) in the gas phase above the solution with molar fraction of toluene 30% at its boiling point Tbp. ____________ D) Approximate molar fraction of solvent which remains in the liquid phase upon heating of mixture with 60% of toluene to 100oC. _______________The table below shows temperature/composition data collected for a mixture of methylbenzene (M) and octane (O) at 1 atm. Recall that x stands for the mole fraction in the liquid and y stands for the mole fraction in the vapor in equilibrium. The boiling points for methylbenzene (M) and octane (O) are 110.60C and 125.60C, respectively. Construct the phase diagram with Temperature vs. xM. What is the composition of the vapor in equilibrium with the liquid of composition (a) xM = 0.250 and (b) xO = 0.250. T (0C) 110.9 112.0 114.0 115.8 117.3 119.0 121.1 123.0 xM 0.908 0.795 0.615 0.527 0.408 0.300 0.203 0.097 yM 0.923 0.836 0.698 0.624 0.527 0.410 0.297 0.164
- Detailed calculations on how to prepare the solutions listed below Solution: 50 mL 1 M oxalic acid Oxalic acid Mw = 90.03 g mol-1 Oxalic acid purity = 98% Solution: 10 mL 3 M sulfuric acid Sulfuric acid Mw = 98.079 g mol-1 Sulfuric acid density = 1.84 g mL-1 Sulfuric acid purity = 98% Solution: 10 mL saturated potassium oxalate potassium oxalate (monohydrate used) Mw = 184.23 g mol-1 Solubility of potassium oxalate in water at 25°C = 360 mg mL-1 Solution :20 mL 3% hydrogen peroxide Hydrogen peroxide is commercially available as a 32% solution.the dimensionless Henry's law constant for trichloroethylene at 25 degrees Celcius is 0.4. A sealed glass vial is prepared that has an air volume of 4ml overlying an aqueous volume of 36ml. TCE is added to the aqueous phase so that initially it has an aqueous-phase concentration of 100 ppb. After the system equilibrates, what will be the concentration (in units of microgram per liter) of TCE in the aqueous phase(1a) Pure benzophenane freezes at 66.6 °C. A solution of 7.75 g of biphenyl (C12H10; molar mass = 154.2g/mol) plus 200.08 g of benzophenane froze at 59.9 °C. Calculate the Kf of benzophenane in °C*kg/mol. (b) An unknown solution with possible cations (Ag+, Hg22+, Pb2+) is treated with 6M HCl and a white precipitate forms. Hot water is added to the precipitate, and a white solid remains after filtration. The white solid is treated with aqueous NH3, and the precipitate turns grey or black. The resulting solution is treated with HNO3 and no precipitate forms. What cations can you determine to be present, absent or maybe present? Explain your reason





