7.The diagram below is an example of Undistillable Mixtures (T vs Mole Fraction).How many degrees of freedom at the vapor phase,liquid phase and at azeotropic point? Undistillable Mixtures – Azeotropes T= T These types of mixtures cannot be separated using simple distillation processes, because the composition of the liquid phase at the azeotropic point is the same as that of their vapor composition. Vapor Phase T= TR T This type of azeotropic phenomenon is the high - boiling azeotropism. This happens when A and B have strong attractive interaction. Liquid Phase Zazeotrope
7.The diagram below is an example of Undistillable Mixtures (T vs Mole Fraction).How many degrees of freedom at the vapor phase,liquid phase and at azeotropic point? Undistillable Mixtures – Azeotropes T= T These types of mixtures cannot be separated using simple distillation processes, because the composition of the liquid phase at the azeotropic point is the same as that of their vapor composition. Vapor Phase T= TR T This type of azeotropic phenomenon is the high - boiling azeotropism. This happens when A and B have strong attractive interaction. Liquid Phase Zazeotrope
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.84QE
Related questions
Question
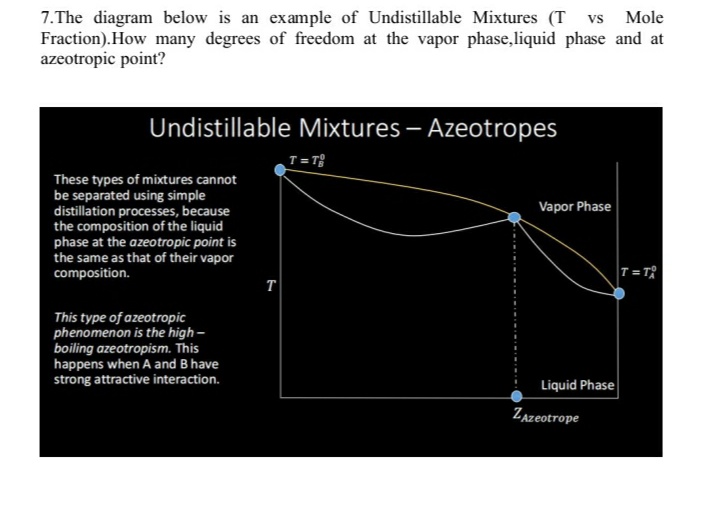
Transcribed Image Text:7.The diagram below is an example of Undistillable Mixtures (T vs Mole
Fraction).How many degrees of freedom at the vapor phase,liquid phase and at
azeotropic point?
Undistillable Mixtures – Azeotropes
T= T
These types of mixtures cannot
be separated using simple
distillation processes, because
the composition of the liquid
phase at the azeotropic point is
the same as that of their vapor
composition.
Vapor Phase
T=T
T
This type of azeotropic
phenomenon is the high -
boiling azeotropism. This
happens when A and B have
strong attractive interaction.
Liquid Phase
Zazeotrope
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,