In his classic studies of stereochemistry and optical activity in organic compounds, Pasteur measured the optical activity of many solutions. For the naturally occurring enantiomer of tartaric acid, [a],20 = +12.4°. What is the % of the (+)-enantiomer in the mixture if the observed rotation is a = -6.0°, with a concentration of 0.54 g/mL in a 2.00 dm polarimeter tube? A. 25.8% B. 27.6% C. 44.8% D. 72.4% E. 74.2% A O B D O E
In his classic studies of stereochemistry and optical activity in organic compounds, Pasteur measured the optical activity of many solutions. For the naturally occurring enantiomer of tartaric acid, [a],20 = +12.4°. What is the % of the (+)-enantiomer in the mixture if the observed rotation is a = -6.0°, with a concentration of 0.54 g/mL in a 2.00 dm polarimeter tube? A. 25.8% B. 27.6% C. 44.8% D. 72.4% E. 74.2% A O B D O E
Basic Clinical Laboratory Techniques 6E
6th Edition
ISBN:9781133893943
Author:ESTRIDGE
Publisher:ESTRIDGE
Chapter6: Basic Clinical Chemistry
Section6.1: Introduction To Clinical Chemistry
Problem 12RQ
Related questions
Question
![In his classic studies of stereochemistry and optical activity in organic compounds,
Pasteur measured the optical activity of many solutions. For the naturally occurring
enantiomer of tartaric acid, [a]p20 = +12.4°. What is the % of the (+)-enantiomer in the
mixture if the observed rotation is a = -6.0°, with a concentration of 0.54 g/mL in a
2.00 dm polarimeter tube?
A. 25.8%
В. 27.6%
C. 44.8%
D. 72.4%
E. 74.2%
А
C
O E
B.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F521b2f63-68c4-44e4-a84c-bf63a3e7922c%2Fc5c37a3c-cccb-4e45-85c7-35f0f0343d87%2Fsendfy4_processed.png&w=3840&q=75)
Transcribed Image Text:In his classic studies of stereochemistry and optical activity in organic compounds,
Pasteur measured the optical activity of many solutions. For the naturally occurring
enantiomer of tartaric acid, [a]p20 = +12.4°. What is the % of the (+)-enantiomer in the
mixture if the observed rotation is a = -6.0°, with a concentration of 0.54 g/mL in a
2.00 dm polarimeter tube?
A. 25.8%
В. 27.6%
C. 44.8%
D. 72.4%
E. 74.2%
А
C
O E
B.
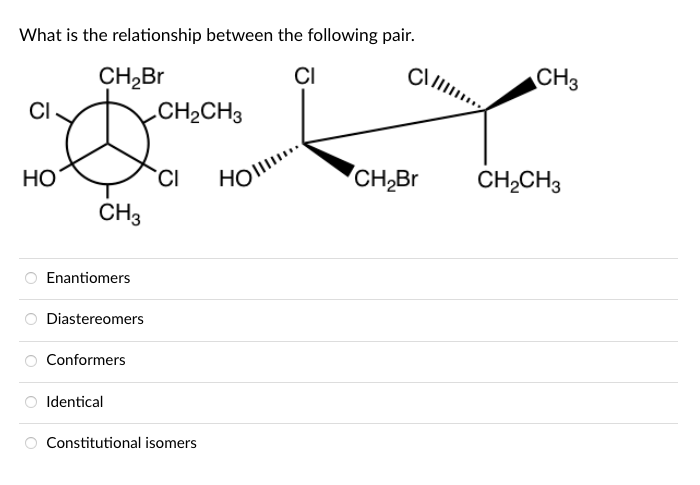
Transcribed Image Text:What is the relationship between the following pair.
CH,Br
CI
CH3
CI
CH2CH3
Но
HOl..
CH,Br
CH2CH3
CH3
Enantiomers
Diastereomers
Conformers
O Identical
O Constitutional isomers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning


Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning