In order to do electron microscopy the samples had to be specially prepared. Were the cells alive at the time of viewing? Explain why you said yes or no
In order to do electron microscopy the samples had to be specially prepared. Were the cells alive at the time of viewing? Explain why you said yes or no
Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter22: Prokaryotes: Bacteria And Archaea
Section: Chapter Questions
Problem 24RQ: A person in England arrives at a medical clinic with a fever and swollen lymph nodes shortly after...
Related questions
Question
In order to do electron microscopy the samples had to be specially prepared. Were the cells alive at the time of viewing? Explain why you said yes or no
I need help answering this queshtion the answer is with the article and it has to be as short as possible
URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC106848/

Transcribed Image Text:The Capsule and S-Layer: Two Independent and Yet
Compatible Macromolecular Structures in Bacillus anthracis
STÉPHANE MESNAGE,¹ EVELYNE TOSI-COUTURE,² PIERRE GOUNON,² MICHÈLE MOCK,¹
AND AGNÈS FOUET¹*
Toxines et Pathogénie Bactériennes (CNRS URA 1858)¹ and Station Centrale de Microscopie Electronique,2
Institut Pasteur, Paris, France
Received 5 September 1997/Accepted 22 October 1997
Bacillus anthracis, the etiological agent of anthrax, is a gram-positive spore-forming bacterium. Fully virulent
bacilli are toxinogenic and capsulated. Two abundant surface proteins, including the major antigen, are
components of the B. anthracis surface layer (S-layer). The B. anthracis paracrystalline S-layer has previously
only been found in noncapsulated vegetative cells. Here we report that the S-layer proteins are also synthesized
under conditions where the poly-y-D-glutamic acid capsule is present. Structural and immunological analyses
show that the capsule is exterior to and completely covers the S-layer proteins. Nevertheless, analysis of single
and double S-layer protein mutants shows that the presence of these proteins is not required for normal
capsulation of the bacilli. Similarly, the S-layer proteins assemble as a two-dimensional crystal, even in the
presence of the capsule. Thus, both structures are compatible, and yet neither is required for the correct
formation of the other.
Bacillus anthracis, a gram-positive spore-forming bacterium,
is the causative agent of anthrax. This disease, to which many
animals, including humans, are susceptible, involves toxemia
and septicemia. In the mammalian host, B. anthracis bacilli
synthesize two toxins (lethal and edema toxins) (31) and a
capsule (18) encoded by two large plasmids, pX01 and pXO2,
respectively (12, 21). The capsule is composed of poly-y-D-
glutamic acid and has antiphagocytic properties (13, 31, 37).
Although unusual, a similar capsule is also found on Bacillus
licheniformis bacilli (9). In the absence of pXO2 or the inducer
bicarbonate, the cell does not produce a capsule and the cell
wall appears layered. These layers are composed of fragments
displaying a highly patterned ultrastructure (10, 16). This type
of cell surface is now referred to as the surface layer (S-layer).
S-layers are present on the surfaces of many archaea and
bacteria (for reviews, see references 29 and 30). Most are
formed by noncovalent, entropy-driven assembly of a single
(glyco)protein protomer on the bacterial surface, giving rise to
proteinaceous paracrystalline layers. Generally, a single S-
layer is present, constituting 5 to 10% of total cell protein. Its
synthesis is thus presumably energy consuming for the bacte-
rium. Numerous bacteria have S-layers, suggesting that they
play important roles in the interaction between the cell and its
environment. Various functions have been proposed for S-
layers, including shape maintenance and molecular sieving,
and they can serve in phage fixation. The S-layer may be a
virulence factor, protecting pathogenic bacteria against com-
plement killing, facilitating binding of bacteria to host mole-
cules, or enhancing their ability to associate with macrophages
(for reviews, see references 27 and 29).
Some bacteria, such as cyanobacteria or Azotobacter spp.,
possess both a capsule and an S-layer; however, to our knowl-
edge, their structural relationships have not been analyzed
through simultaneous genetic and cytologic studies. Both of
these features have been independently described for the sur-
* Corresponding author. Mailing address: Toxines et Pathogénie
Bactériennes, Institut Pasteur, 28, rue du Dr Roux, 75724 Paris Cedex
15, France. Phone: 33 1 45 68 86 54. Fax: 33 1 45 68 89 54. E-mail:
afouet@pasteur.fr.
52
face of the pathogenic bacterium B. anthracis. The components
of the B. anthracis S-layer are two abundant surface proteins,
EA1 and Sap (6, 20). Previous analyses of the B. anthracis
S-layer used plasmid-cured strains; consequently, the interac-
tion, if any, between the capsule and the S-layer could not be
studied. Temporal or environmental regulation could be such
that only one or the other structure is ever present at the cell
surface. However, we show that S-layer proteins are synthe-
sized under conditions where the bacilli are capsulated. We
determined the localizations of capsule and S-layer compo-
nents and analyzed whether the S-layer is necessary for proper
capsulation. Finally, the assembly of the S-layer proteins in a
two-dimensional crystal was examined in the presence of the
capsule.
MATERIALS AND METHODS
Plasmids, bacterial strains, mating experiments, and culture conditions. The
plasmids used to disrupt sap (encoding Sap), eag (encoding EA1), and both
genes, i.e., pEAI207, pSAL322, and pSAL303, respectively, were described pre-
viously (6, 20) and are listed in Table 1. The construction of B. anthracis CAF10,
pXO2 transductant of plasmidless strain 9131, and its
synthesis have already been reported (8). Escherichia coli JM83 harboring
pRK24 was used for mating experiments (34, 35). Allelic exchange was carried
out as previously described (26) with the spectinomycin resistance cassette as
selectable marker (24). sap, eag, and both genes were disrupted in CAF10 by
heterogramic conjugation, giving CBA91, CSM91, and CSM11, respectively (Ta-
ble 1). E. coli cells were grown in Luria broth or on L agar plates (22). Capsule
synthesis was induced by growing B. anthracis cells in brain heart infusion me-
dium (Difco Laboratories) in the presence of 0.6% sodium bicarbonate
CAP plates (28) in a 5% CO₂ atmosphere for electron microscopy. Antibiotics
were used at the following concentrations: kanamycin, 40 µg/ml for E. coli;
erythromycin, 5 µg/ml for B. anthracis; and spectinomycin, 60 µg/ml for both E.
coli and B. anthracis.
on
Protein
analysis. To test the in vivo expression of EA1 and Sap, the synthesis
of antibodies was assayed. The rationale of this experiment is that antibodies are
produced only if the antigen is synthesized in vivo. Seven Swiss mice were
injected with 106 spores of strain CAF10 and sacrificed after 30 days. Their sera
were pooled. The gel loading samples were obtained as follows. B. anthracis cells
were harvested at an optical density at 600 nm of =2. Pellets were washed in 125
mM Tris-HCl (pH 8.0), sonicated until complete clarification, and resuspended
in Laemmli buffer (19). Samples (3 µg of pellet protein and 20 μl of trichloro-
acetic acid-precipitated supernatant protein) were loaded on sodium dodecyl
sulfate-10% polyacrylamide gels. Separated proteins were transferred to nitro-
cellulose sheets by use of the Bio-Rad Trans-Blot system. The sera were used at
a 1/200 dilution. Western blots were developed with the ECL Western blotting
analysis system (Amersham), with a 1/10,000 dilution of the second antibody.
Transcribed Image Text:Capsule observation. The aspect and homogeneity of capsulation were
checked by India ink exclusion (4).
Electron microscopy. (i) Thin sections. Cells were fixed with 2% formaldehyde
(made freshly from paraformaldehyde) and 2.5% glutaraldehyde in 0.1 M caco-
dylate buffer (pH 7.2) containing 5 mM CaCl₂ (14, 17). After being washed, the
cells were postfixed for 2 h with 2% OsO4 in the same buffer. The pelleted.
bacteria were embedded in 2% low-melting-point agar (type IX; Sigma) (36).
The samples were then treated for 16 h with 0.5% uranyl acetate in water. After
extensive washing, small blocks were dehydrated with alcohol and embedded in
Spurr's medium (Ladd Inc.) (32). Thin sections were stained conventionally and
observed with a Philips CM12 electron microscope.
(ii) Immunocytochemistry with thin sections. B. anthracis cells were fixed with
2% formaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate-buffered saline
(14 mM Na₂HPO4, 7 mM NaH₂PO4, 150 mM NaCl) (PBS) (pH 7.4) for 1 h,
rinsed in the same buffer, and embedded in 2% low-melting-point agar (36).
Small blocks containing bacteria were embedded in Lowicryl HM20 (Poly-
sciences Ltd.) at -50°C following the progressively lower temperatures protocol
of Carlemalm et al. (3) as described by Newman and Hobot (25). Thin sections
were collected onto Formvar-carbon-coated nickel grids and incubated succes-
sively at room temperature with the following solutions: PBS-50 mM NH4Cl for
10 min; PBS-1% bovine serum albumin (BSA) -1% normal goat serum-0.1%
Tween 20 for 10 min; specific anti-EA1 or anti-Sap antibodies diluted 1/50 in
PBS-1% BSA-1% normal goat serum-0.1% Tween 20 for 1 h; PBS-0.1% BSA
three times for 5 min each time; goat immunoglobulin G (heavy and light chains)
anti-rabbit immunoglobulin-gold conjugate diluted 1/20 in PBS-0.01% gelatin
08
54 MESNAGE ET AL.
A
B
for 1 h; PBS three times for 5 min each time; PBS-1% glutaraldehyde for 5 min;
and five times with water. The thin sections were then stained by incubation with
2% uranyl acetate in water for 35 min and then in lead tartrate for 2 min (23).
(iii) Immunocytochemistry with whole-mount cells. Immunocytochemistry
with whole-mount cells was carried out as previously described (20).
(iv) Negative staining experiments. B. anthracis cells were resuspended in a
1/10 volume of 25 mM Tris-HCl (pH 8.0)-10 mM MgCl₂ with 0.25 or 0.5%
glutaraldehyde for EA1 or Sap, respectively, in the presence of approximately 30
μl of 425- to 600-μm glass beads (Sigma) and disrupted by vortexing for 30 s. This
treatment disintegrated the capsule. Negative staining was performed as previ-
ously described (20). Micrographs were recorded with a Philips CM12 electron
microscope under low-dose (17 electrons/Å/s) transmission electron microscopy
conditions.
A
B
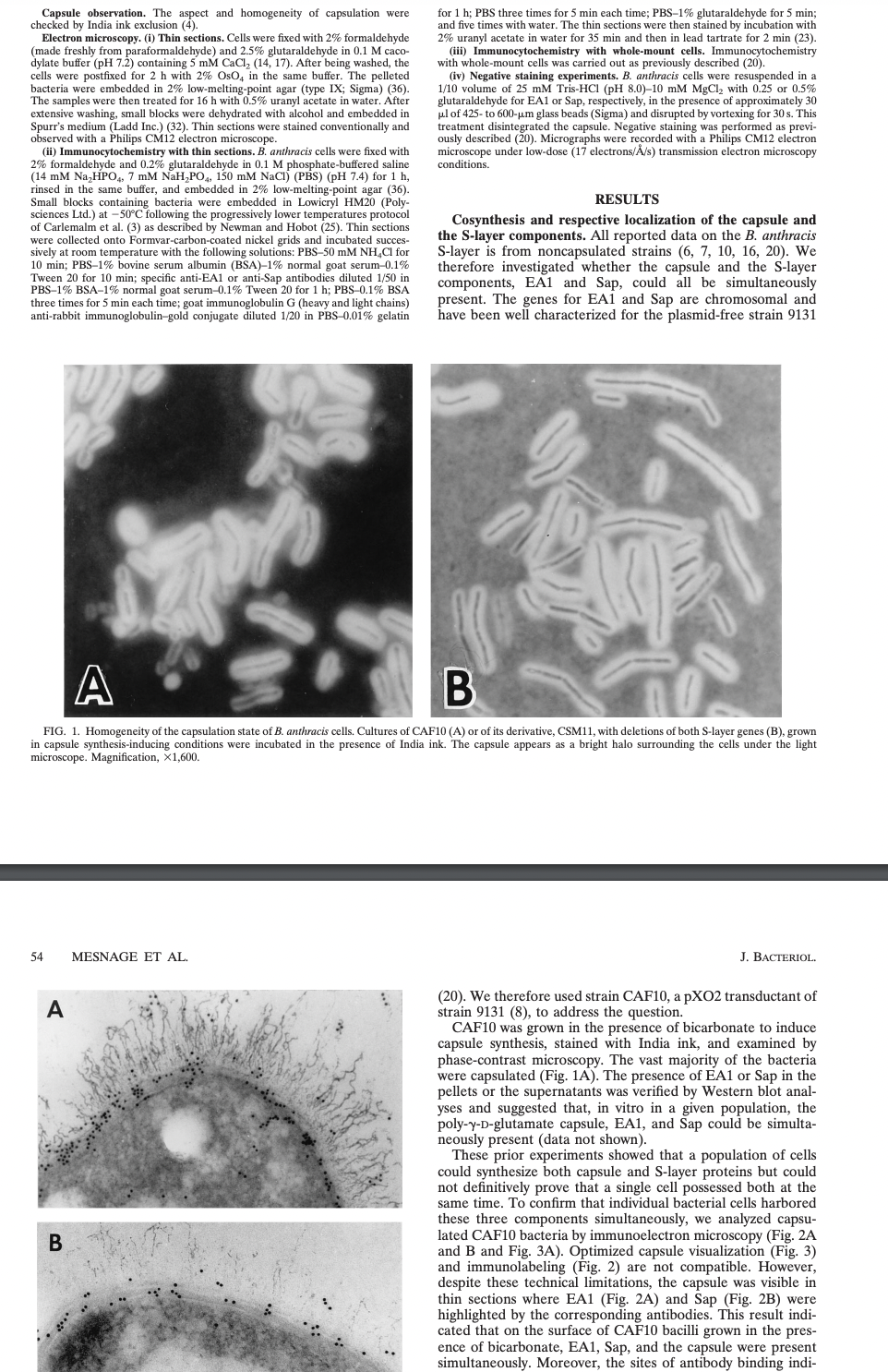
FIG. 1. Homogeneity of the capsulation state of B. anthracis cells. Cultures of CAF10 (A) or of its derivative, CSM11, with deletions of both S-layer genes (B), grown
in capsule synthesis-inducing conditions were incubated in the presence of India ink. The capsule appears as a bright halo surrounding the cells under the light
microscope. Magnification, X1,600.
RESULTS
Cosynthesis and respective localization of the capsule and
the S-layer components. All reported data on the B. anthracis
S-layer is from noncapsulated strains (6, 7, 10, 16, 20). We
therefore investigated whether the capsule and the S-layer
components, EA1 and Sap, could all be simultaneously
present. The genes for EA1 and Sap are chromosomal and
have been well characterized for the plasmid-free strain 9131
J. BACTERIOL.
(20). We therefore used strain CAF10, a pXO2 transductant of
strain 9131 (8), to address the question.
CAF10 was grown in the presence of bicarbonate to induce
capsule synthesis, stained with India ink, and examined by
phase-contrast microscopy. The vast majority of the bacteria
were capsulated (Fig. 1A). The presence of EA1 or Sap in the
pellets or the supernatants was verified by Western blot anal-
yses and suggested that, in vitro in a given population, the
poly-y-D-glutamate capsule, EA1, and Sap could be simulta-
neously present (data not shown).
These prior experiments showed that a population of cells
could synthesize both capsule and S-layer proteins but could
not definitively prove that a single cell possessed both at the
same time. To confirm that individual bacterial cells harbored
these three components simultaneously, we analyzed capsu-
lated CAF10 bacteria by immunoelectron microscopy (Fig. 2A
and B and Fig. 3A). Optimized capsule visualization (Fig. 3)
and immunolabeling (Fig. 2) are not compatible. However,
despite these technical limitations, the capsule was visible in
thin sections where EA1 (Fig. 2A) and Sap (Fig. 2B) were
highlighted by the corresponding antibodies. This result indi-
cated that on the surface of CAF10 bacilli grown in the pres-
ence of bicarbonate, EA1, Sap, and the capsule were present
simultaneously. Moreover, the sites of antibody binding indi-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax


Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax
