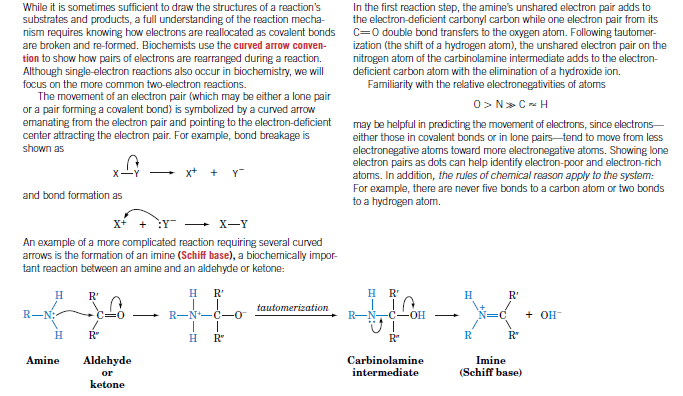
In the first reaction step, the amine's unshared electron pair adds to the electron-deficient carbonyl carbon while one electron pair from its C=0 double bond transfers to the oxygen atom. Following tautomer- ization (the shift of a hydrogen atom), the unshared electron pair on the nitrogen atom of the carbinolamine intermediate adds to the electron- deficient carbon atom with the elimination of a hydroxide ion. Familiarity with the relative electronegativities of atoms While it is sometimes sufficient to draw the structures of a reaction's substrates and products, a full understanding of the reaction mecha- nism requires knowing how electrons are reallocated as covalent bonds are broken and re-formed. Biochemists use the curved arrow conven- tion to show how pairs of electrons are rearranged during a reaction. Although single-electron reactions also occur in biochemistry, we wll focus on the more common two-electron reactions. The moverment of an electron pair (which may be either a lone pair or a pair forming a covalent bond) is symbolized by a curved arrow emanating from the electron pair and pointing to the electron-deficient center attracting the electron pair. For example, bond breakage is O> N» C- H may be helpful in predicting the movement of electrons, since electrons- either those in covalent bonds or in lone pairs tend to move from less electronegative atoms toward more electronegative atoms. Showing lone electron pairs as dots can help identify electron-poor and electron-rich atoms. In addition, the rules of chemical reason apply to the system: For example, there are never five bonds to a carbon atom or two bonds to a hydrogen atom. shown as x+ + Y and bond formation as X-Y An example of a more complicated reaction requiring several curved arrows is the formation of an imine (Schiff base), a biochemically impor- tant reaction between an amine and an aldehyde or ketone: H R' H R' H R H R' tautomerization R-N; + R-N*-C- R-N,C-oH + OH H R" H R" R" R R' Amine Aldehyde Carbinolamine intermediate Imine (Schiff base) or ketone
In the first reaction step, the amine's unshared electron pair adds to the electron-deficient carbonyl carbon while one electron pair from its C=0 double bond transfers to the oxygen atom. Following tautomer- ization (the shift of a hydrogen atom), the unshared electron pair on the nitrogen atom of the carbinolamine intermediate adds to the electron- deficient carbon atom with the elimination of a hydroxide ion. Familiarity with the relative electronegativities of atoms While it is sometimes sufficient to draw the structures of a reaction's substrates and products, a full understanding of the reaction mecha- nism requires knowing how electrons are reallocated as covalent bonds are broken and re-formed. Biochemists use the curved arrow conven- tion to show how pairs of electrons are rearranged during a reaction. Although single-electron reactions also occur in biochemistry, we wll focus on the more common two-electron reactions. The moverment of an electron pair (which may be either a lone pair or a pair forming a covalent bond) is symbolized by a curved arrow emanating from the electron pair and pointing to the electron-deficient center attracting the electron pair. For example, bond breakage is O> N» C- H may be helpful in predicting the movement of electrons, since electrons- either those in covalent bonds or in lone pairs tend to move from less electronegative atoms toward more electronegative atoms. Showing lone electron pairs as dots can help identify electron-poor and electron-rich atoms. In addition, the rules of chemical reason apply to the system: For example, there are never five bonds to a carbon atom or two bonds to a hydrogen atom. shown as x+ + Y and bond formation as X-Y An example of a more complicated reaction requiring several curved arrows is the formation of an imine (Schiff base), a biochemically impor- tant reaction between an amine and an aldehyde or ketone: H R' H R' H R H R' tautomerization R-N; + R-N*-C- R-N,C-oH + OH H R" H R" R" R R' Amine Aldehyde Carbinolamine intermediate Imine (Schiff base) or ketone
Chapter23: Carbonyl Condensation Reactions
Section23.SE: Something Extra
Problem 45MP
Related questions
Question
Using the reaction shown in Box (the attack of an
Transcribed Image Text:In the first reaction step, the amine's unshared electron pair adds to
the electron-deficient carbonyl carbon while one electron pair from its
C=0 double bond transfers to the oxygen atom. Following tautomer-
ization (the shift of a hydrogen atom), the unshared electron pair on the
nitrogen atom of the carbinolamine intermediate adds to the electron-
deficient carbon atom with the elimination of a hydroxide ion.
Familiarity with the relative electronegativities of atoms
While it is sometimes sufficient to draw the structures of a reaction's
substrates and products, a full understanding of the reaction mecha-
nism requires knowing how electrons are reallocated as covalent bonds
are broken and re-formed. Biochemists use the curved arrow conven-
tion to show how pairs of electrons are rearranged during a reaction.
Although single-electron reactions also occur in biochemistry, we wll
focus on the more common two-electron reactions.
The moverment of an electron pair (which may be either a lone pair
or a pair forming a covalent bond) is symbolized by a curved arrow
emanating from the electron pair and pointing to the electron-deficient
center attracting the electron pair. For example, bond breakage is
O> N» C- H
may be helpful in predicting the movement of electrons, since electrons-
either those in covalent bonds or in lone pairs tend to move from less
electronegative atoms toward more electronegative atoms. Showing lone
electron pairs as dots can help identify electron-poor and electron-rich
atoms. In addition, the rules of chemical reason apply to the system:
For example, there are never five bonds to a carbon atom or two bonds
to a hydrogen atom.
shown as
x+
+
Y
and bond formation as
X-Y
An example of a more complicated reaction requiring several curved
arrows is the formation of an imine (Schiff base), a biochemically impor-
tant reaction between an amine and an aldehyde or ketone:
H
R'
H
R'
H R
H
R'
tautomerization
R-N;
+ R-N*-C-
R-N,C-oH
+ OH
H
R"
H
R"
R"
R
R'
Amine
Aldehyde
Carbinolamine
intermediate
Imine
(Schiff base)
or
ketone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning