In the given acid-base titration curve, what is the analyte being titrated ? 14 12 10 8 6. 4 2 10 15 20 25 30 35 40 45 Volume of 0.1 M NaOH added in mL strong acid weak base strong base O weak acid 00 Hd
In the given acid-base titration curve, what is the analyte being titrated ? 14 12 10 8 6. 4 2 10 15 20 25 30 35 40 45 Volume of 0.1 M NaOH added in mL strong acid weak base strong base O weak acid 00 Hd
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section15.2: Acid-base Titrations
Problem 15.6E: Draw the titration curve for the titration of 50.0 mL of 0.100-M NaOH using 0.100-M HCl as the...
Related questions
Question
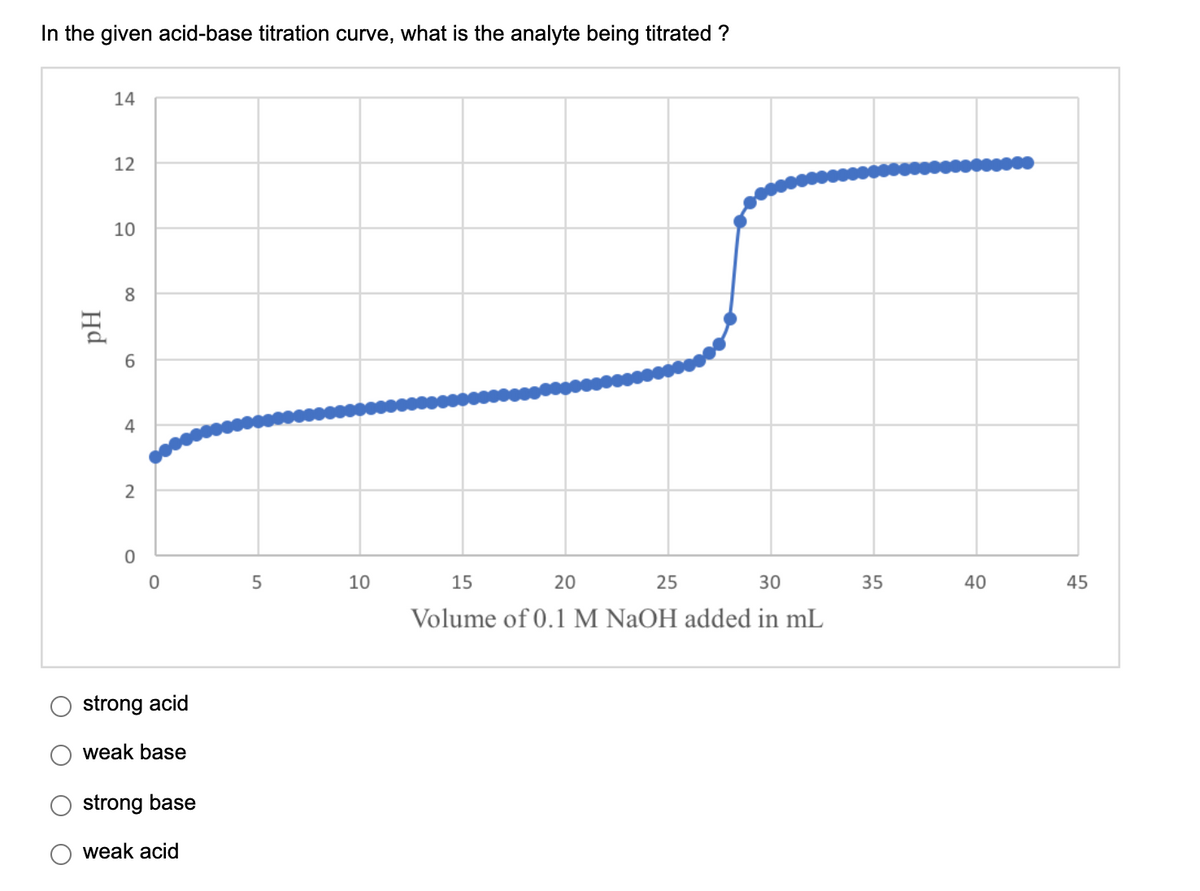
Transcribed Image Text:In the given acid-base titration curve, what is the analyte being titrated ?
14
12
10
8.
6
4
10
15
20
25
30
35
40
45
Volume of 0.1 M NaOH added in mL
strong acid
weak base
strong base
weak acid
Hd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole