In the series of group 16 hydrides, of general formula MH2, the measured bond distances are 0-H, 96 pm; S-H, 134 pm; Se-H, 146 pm. (a) Compare these values with those estimated by use of the atomic radii in Figure 7.7. (b) Explain the steady increase in M-H bond dis- tance in this series in terms of the electron configurations of the Matoms.
In the series of group 16 hydrides, of general formula MH2, the measured bond distances are 0-H, 96 pm; S-H, 134 pm; Se-H, 146 pm. (a) Compare these values with those estimated by use of the atomic radii in Figure 7.7. (b) Explain the steady increase in M-H bond dis- tance in this series in terms of the electron configurations of the Matoms.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter20: The Representative Elements
Section: Chapter Questions
Problem 118IP: Although nitrogen trifluoride (NF3) is a thermally stable compound, nitrogen triiodide (Nl3) is...
Related questions
Question
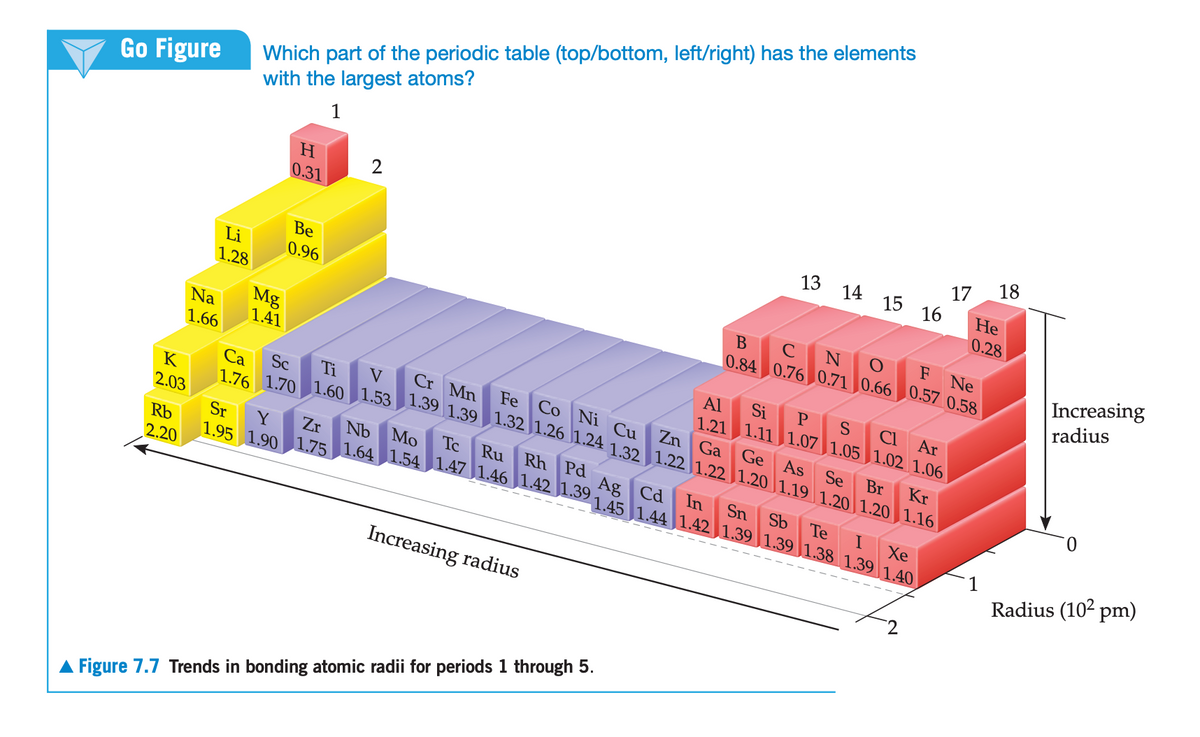
Transcribed Image Text:Which part of the periodic table (top/bottom, left/right) has the elements
with the largest atoms?
Go Figure
1
H.
0.31
2
18
17
13
14
15
16
He
Be
0.96
0.28
Li
1.28
F
Ne
10.84 0.76 0.71 0.66 0.57 0.58
Increasing
radius
Mg
1.41
Na
1.66
Si
Al
1.21 1.11 1.07 |1.05 1.02 1.06
Zn Ga
S
CI
Ar
Ti
V
Cr Mn
Co Ni Cu
ca 70 1.60 1.53 1.39 1.39 1.32|1.26 |1.24 1.32 1.22 1.22 1.20 1.19 1.20 1.20 1.16
Са
Sc
Fe
Ge
As
Se
Br
Kr
2.03
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh Pd
Rb
1.95 1.90 1.75 1.64 1.54 1.47 1.46 1.42 1.39
Ag
Cd
In
Sn
Sb
1.45 1.44 1.42 1.39 1.39 1.38 1.39 1.40
Te
2.20
Xe
Radius (102 pm)
Increasing radius
A Figure 7.7 Trends in bonding atomic radii for periods 1 through 5.

Transcribed Image Text:7.83 In the series of group 16 hydrides, of general formula
MH2, the measured bond distances are 0-H, 96 pm;
S-H, 134 pm; Se-H, 146 pm. (a) Compare these values
with those estimated by use of the atomic radii in Figure
7.7. (b) Explain the steady increase in M-H bond dis-
tance in this series in terms of the electron configurations
of the M atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning