In this task, you'll observe a chemical reaction between baking soda and vinegar. How do you think the temperature of the reactants and the rate of reaction are related? Write a hypothesis to predict the relationship between the two parameters.
In this task, you'll observe a chemical reaction between baking soda and vinegar. How do you think the temperature of the reactants and the rate of reaction are related? Write a hypothesis to predict the relationship between the two parameters.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter20: Rates Of Chemical Reactions, I. The Iodination Of Acetone
Section: Chapter Questions
Problem 3ASA
Related questions
Question
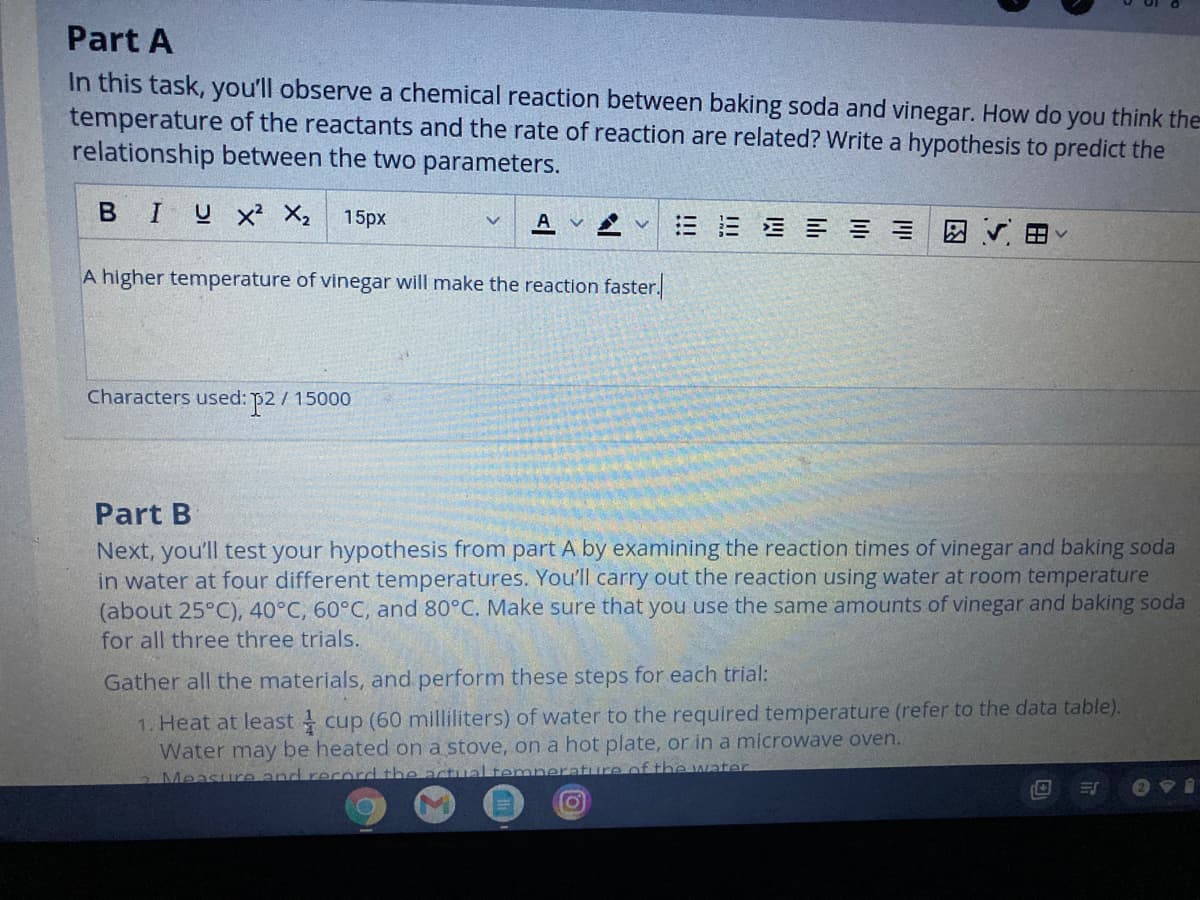
Transcribed Image Text:Part A
In this task, you'll observe a chemical reaction between baking soda and vinegar. How do
temperature of the reactants and the rate of reaction are related? Write a hypothesis to predict the
relationship between the two parameters.
you
think the
в I
U x² X2
15px
E E E E E E r
A
A higher temperature of vinegar will make the reaction faster.
Characters used: p2/ 15000
Part B
Next, you'll test your hypothesis from part A by examining the reaction times of vinegar and baking soda
in water at four different temperatures. You'll carry out the reaction using water at room temperature
(about 25°C), 40°C, 60°C, and 80°C. Make sure that you use the same amounts of vinegar and baking soda
for all three three trials.
Gather all the materials, and perform these steps for each trial:
1. Heat at least cup (60 milliliters) of water to the required temperature (refer to the data table).
Water may be heated on a stove, on a hot plate, or in a microwave oven.
Measure and red
he actual temnerature of the water
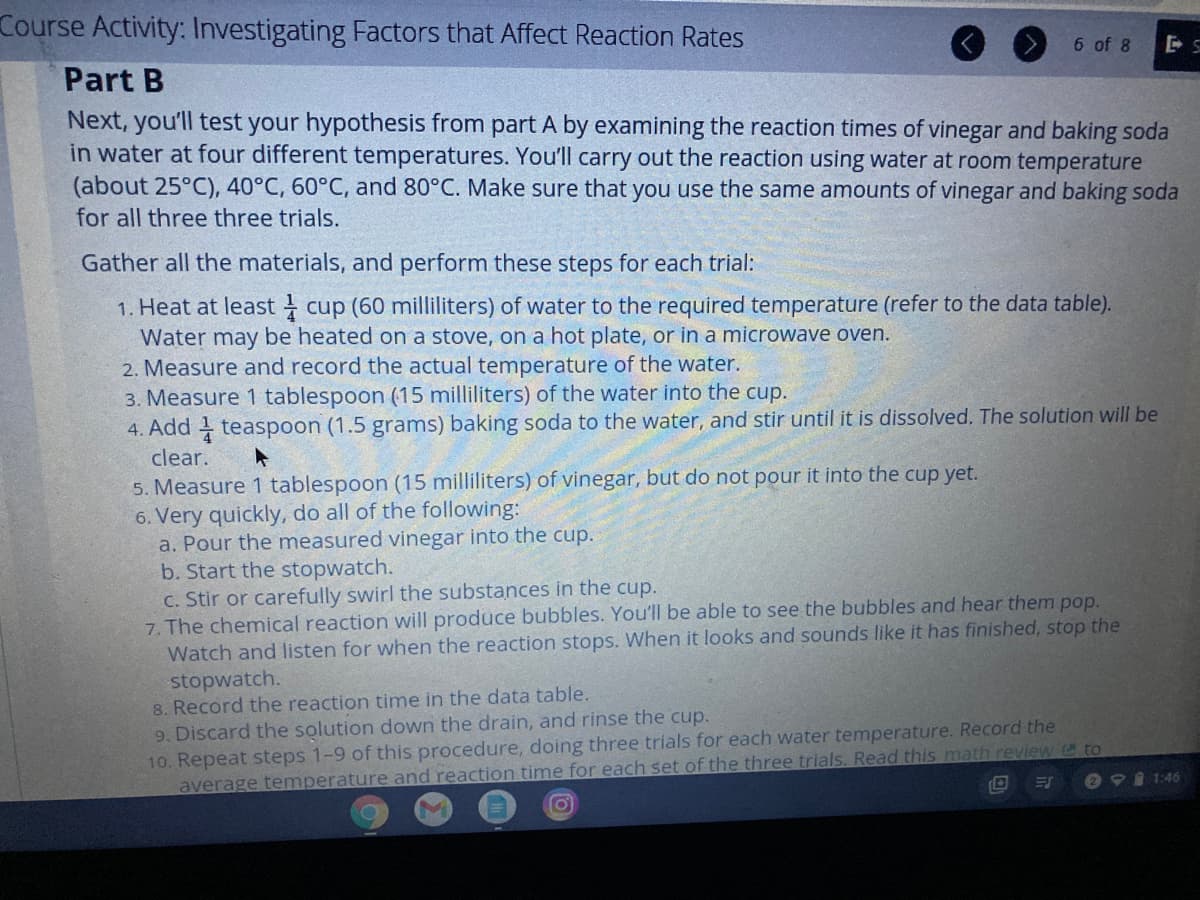
Transcribed Image Text:Course Activity: Investigating Factors that Affect Reaction Rates
6 of 8
Part B
Next, you'll test your hypothesis from part A by examining the reaction times of vinegar and baking soda
in water at four different temperatures. You'll carry out the reaction using water at room temperature
(about 25°C), 40°C, 60°C, and 80°C. Make sure that you use the same amounts of vinegar and baking soda
for all three three trials.
Gather all the materials, and perform these steps for each trial:
1. Heat at least cup (60 milliliters) of water to the required temperature (refer to the data table).
Water may be heated on a stove, on a hot plate, or in a microwave oven.
2. Measure and record the actual temperature of the water.
3. Measure 1 tablespoon (15 milliliters) of the water into the cup.
4. Add teaspoon (1.5 grams) baking soda to the water, and stir until it is dissolved. The solution will be
clear.
5. Measure 1 tablespoon (15 milliliters) of vinegar, but do not pour it into the cup yet.
6. Very quickly, do all of the following:
a. Pour the measured vinegar into the cup.
b. Start the stopwatch.
C. Stir or carefully swirl the substances in the cup.
7. The chemical reaction will produce bubbles. You'll be able to see the bubbles and hear them pop.
Watch and listen for when the reaction stops. When it looks and sounds like it has finished, stop the
stopwatch.
8. Record the reaction time in the data table.
9. Discard the solution down the drain, and rinse the cup.
10. Repeat steps 1-9 of this procedure, doing three trials for each water temperature. Record the
average temperature and reaction time for each set of the three trials. Read this math review @to
e 9I 1:46
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning