"Two chemicals A and B are combined to form a chemical C. The rate of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C. Initially there are 49 grams of A and 68 grams of B, and for each gram of B, 0.8 grams of A is used. It has been observed that 29.25 grams of C is formed in 5 minutes. How much is formed in 30 minutes? What is the limiting amount of C after a long time ? grams of C are formed in 30 minutes grams is the limiting amount of C after long time
"Two chemicals A and B are combined to form a chemical C. The rate of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C. Initially there are 49 grams of A and 68 grams of B, and for each gram of B, 0.8 grams of A is used. It has been observed that 29.25 grams of C is formed in 5 minutes. How much is formed in 30 minutes? What is the limiting amount of C after a long time ? grams of C are formed in 30 minutes grams is the limiting amount of C after long time
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.2E: The oxidation-reduction reaction between iron metal and aqueous permanganate ions in acidic solution...
Related questions
Question
100%
Please answer
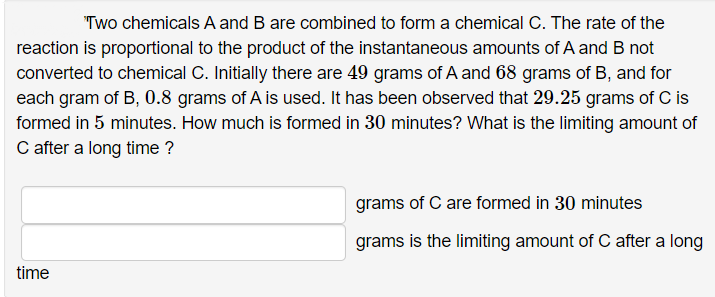
Transcribed Image Text:"Two chemicals A and B are combined to form a chemical C. The rate of the
reaction is proportional to the product of the instantaneous amounts of A and B not
converted to chemical C. Initially there are 49 grams of A and 68 grams of B, and for
each gram of B, 0.8 grams of A is used. It has been observed that 29.25 grams of C is
formed in 5 minutes. How much is formed in 30 minutes? What is the limiting amount of
C after a long time ?
grams of C are formed in 30 minutes
grams is the limiting amount of C after a long
time
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
