created Is I, being created or destroyed by the chemical reaction? O destroyed neither created nor destroyed If I, is being created or destroyed, what is the rate at which it is being created or destroyed 80 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If I, is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 80 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.
created Is I, being created or destroyed by the chemical reaction? O destroyed neither created nor destroyed If I, is being created or destroyed, what is the rate at which it is being created or destroyed 80 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If I, is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 80 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.12E: 5.12. True or false: If all the partial pressures of reactants and products drop by half, the value...
Related questions
Question
100%
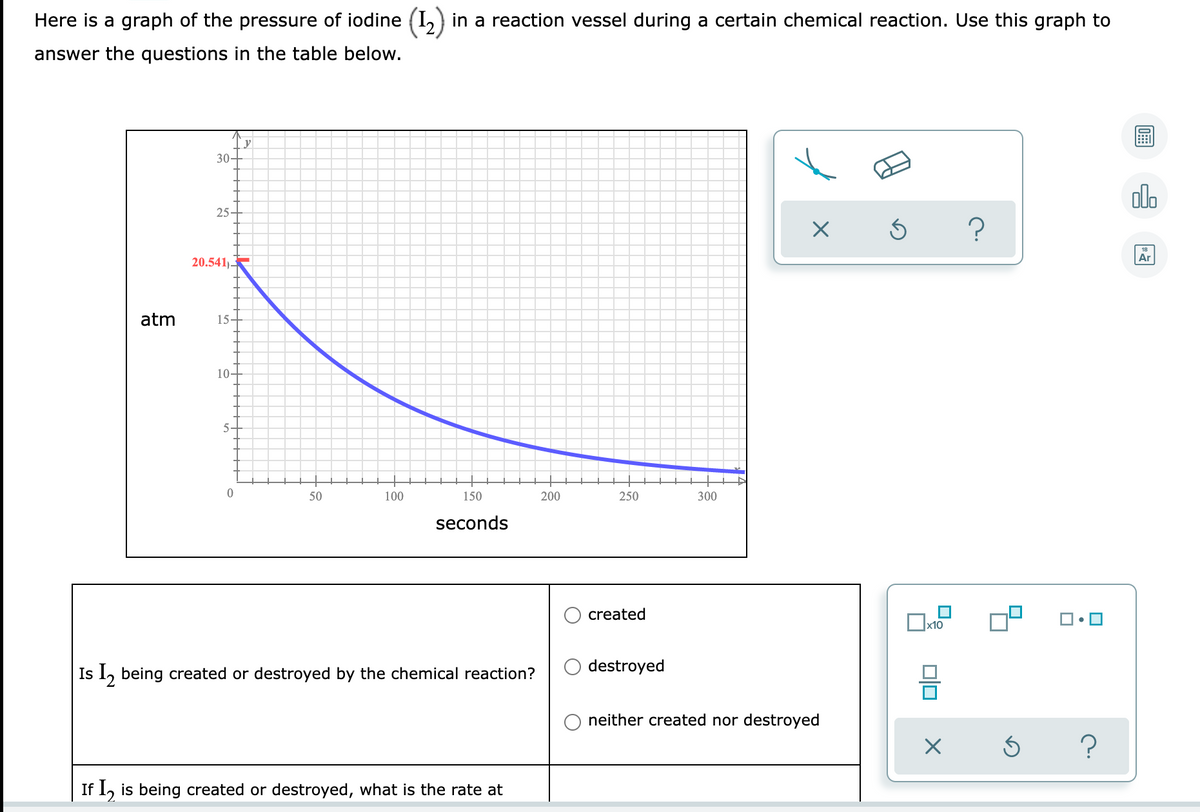
Transcribed Image Text:Here is a graph of the pressure of iodine (I,) in a reaction vessel during a certain chemical reaction. Use this graph to
answer the questions in the table below.
30-
olo
25
Ar
20.541)-
atm
15-
10-
5-
50
100
150
200
250
300
seconds
created
destroyed
Is 1, being created or destroyed by the chemical reaction?
neither created nor destroyed
If 1, is being created or destroyed, what is the rate at
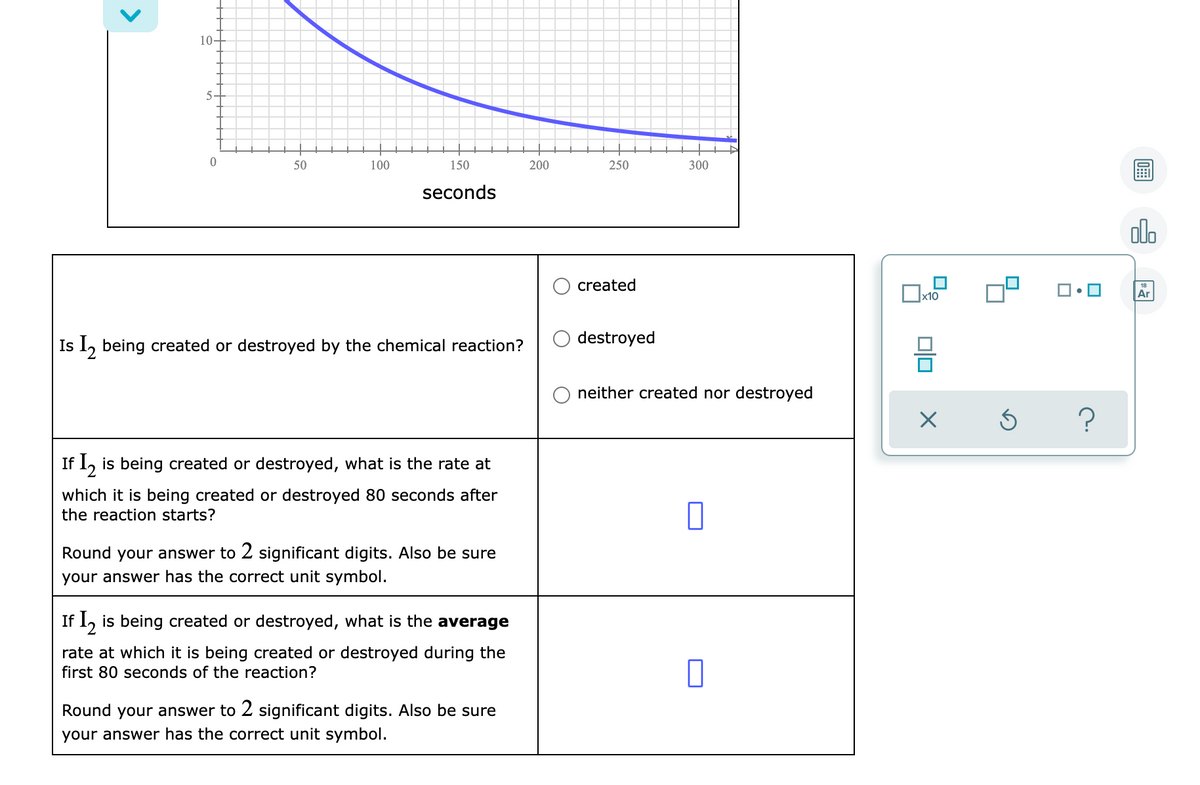
Transcribed Image Text:10-
50
100
150
200
250
300
seconds
olo
created
Ar
Is l, being created or destroyed by the chemical reaction?
destroyed
neither created nor destroyed
If 1, is being created or destroyed, what is the rate at
which it is being created or destroyed 80 seconds after
the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If 1, is being created or destroyed, what is the average
rate at which it is being created or destroyed during the
first 80 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning