In this virtual lab, calorimetry will be performed to determine the heat 100 capacity, Ceal , of a coffee cup calorimeter. Imagine that, in lab the mass of a piece of iron, mre, is recorded as mfe = 51.56 g In the next step of the lab (as shown in the image) the piece of metal is heated up. Record the metal's highest temperature, Te. (Read to the tenths place) Tre = 94.6 °C Next, the metal is dropped into the coffee cup calorimeter. Please continue to the next image. In another step of this lab, the mass of ethanol (methanol ) in a coffee cup is recorded as methanol = 63.87 g The initial temperature of the ethanol, Tethanol1, is given to be 14.00 °C. In the next step of the lab (as shown in the image) the hot piece of iron is placed into the cool ethanol. Record the final temperature of the ethanol. Note this also serves as the final temperature of the metal. In the final step, using the specific heats (2.420 and 0.4498 J/(g · °C) for ethanol and iron, respectively) and the mass of ethanol and iron given, calculate the heat, taken up by the ethanol, qethanol , and the heat given off by the metal, qiron , and record them. Tethanol 2 = 24.0 1546 Gethanol = 1637 gFe = Incorrect Suppose a second trial is conducted using iron and ethanol as described. The results from this trial are calculated, yielding a 9ethanol value of 1565 J and a qr, value of –1633 J. Using these results, calculate the heat absorbed by the calorimeter. Ical = Incorrect
In this virtual lab, calorimetry will be performed to determine the heat 100 capacity, Ceal , of a coffee cup calorimeter. Imagine that, in lab the mass of a piece of iron, mre, is recorded as mfe = 51.56 g In the next step of the lab (as shown in the image) the piece of metal is heated up. Record the metal's highest temperature, Te. (Read to the tenths place) Tre = 94.6 °C Next, the metal is dropped into the coffee cup calorimeter. Please continue to the next image. In another step of this lab, the mass of ethanol (methanol ) in a coffee cup is recorded as methanol = 63.87 g The initial temperature of the ethanol, Tethanol1, is given to be 14.00 °C. In the next step of the lab (as shown in the image) the hot piece of iron is placed into the cool ethanol. Record the final temperature of the ethanol. Note this also serves as the final temperature of the metal. In the final step, using the specific heats (2.420 and 0.4498 J/(g · °C) for ethanol and iron, respectively) and the mass of ethanol and iron given, calculate the heat, taken up by the ethanol, qethanol , and the heat given off by the metal, qiron , and record them. Tethanol 2 = 24.0 1546 Gethanol = 1637 gFe = Incorrect Suppose a second trial is conducted using iron and ethanol as described. The results from this trial are calculated, yielding a 9ethanol value of 1565 J and a qr, value of –1633 J. Using these results, calculate the heat absorbed by the calorimeter. Ical = Incorrect
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.109QP: A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter...
Related questions
Question
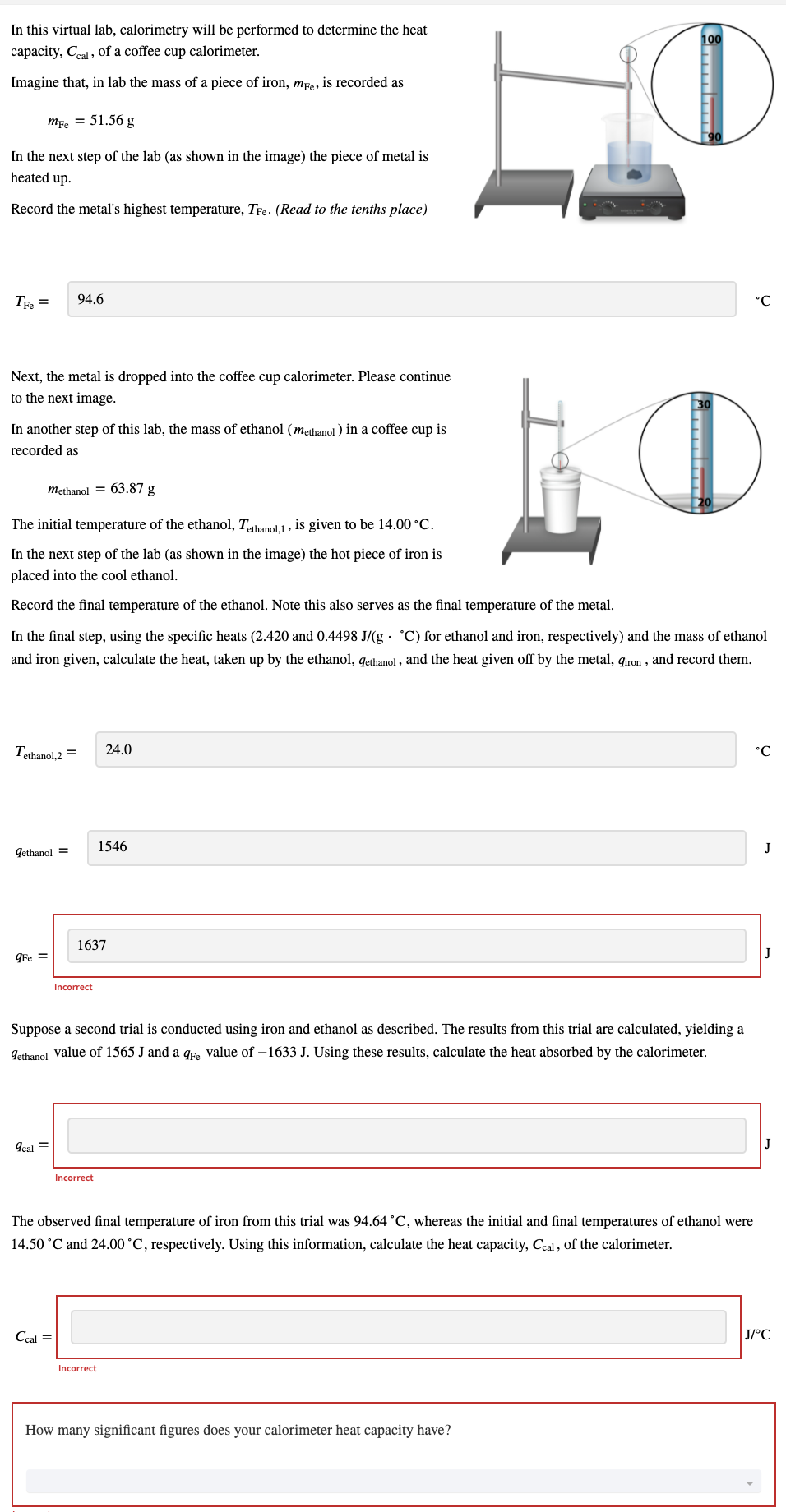
Transcribed Image Text:In this virtual lab, calorimetry will be performed to determine the heat
capacity, Ceal , of a coffee cup calorimeter.
100
Imagine that, in lab the mass of a piece of iron, mpe, is recorded as
Тfe 3 51.56 g
In the next step of the lab (as shown in the image) the piece of metal is
heated up.
Record the metal's highest temperature, Tfe . (Read to the tenths place)
Tre =
94.6
°C
Next, the metal is dropped into the coffee cup calorimeter. Please continue
to the next image.
In another step of this lab, the mass of ethanol (methanol ) in a coffee cup is
recorded as
methanol = 63.87 g
The initial temperature of the ethanol, Tethanol.1, is given to be 14.00 °C.
In the next step of the lab (as shown in the image) the hot piece of iron is
placed into the cool ethanol.
Record the final temperature of the ethanol. Note this also serves as the final temperature of the metal.
In the final step, using the specific heats (2.420 and 0.4498 J/(g · °C) for ethanol and iron, respectively) and the mass of ethanol
and iron given, calculate the heat, taken up by the ethanol, qethanol , and the heat given off by the metal, qiron , and record them.
Tethanol,2 =
24.0
°C
1546
J
qethanol =
1637
J
qFe =
Incorrect
Suppose a second trial is conducted using iron and ethanol as described. The results from this trial are calculated, yielding a
gethanol Value of 1565 J and a gre value of –1633 J. Using these results, calculate the heat absorbed by the calorimeter.
Ical =
J
Incorrect
The observed final temperature of iron from this trial was 94.64°C, whereas the initial and final temperatures of ethanol were
14.50 °C and 24.00 °C, respectively. Using this information, calculate the heat capacity, Ccal , of the calorimeter.
Ccal =
J/°C
Incorrect
How many significant figures does your calorimeter heat capacity have?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning