in Universe L, recently discovered by an intrepid team of chemists who also happen to have studied interdimensional travel, quantum mechanics works just as it does in our universe, except that there are seven d orbitals instead of the usual number we observe here. Jse these facts to write the ground-state electron configurations of the thirteenth and fourteenth elements in the first transition series in Universe L. Vote: you may use X to stand for the electron configuration of the noble gas at the end of the row before the first transition series. thirteenth transition metal: fourteenth transition metal:
in Universe L, recently discovered by an intrepid team of chemists who also happen to have studied interdimensional travel, quantum mechanics works just as it does in our universe, except that there are seven d orbitals instead of the usual number we observe here. Jse these facts to write the ground-state electron configurations of the thirteenth and fourteenth elements in the first transition series in Universe L. Vote: you may use X to stand for the electron configuration of the noble gas at the end of the row before the first transition series. thirteenth transition metal: fourteenth transition metal:
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 103E
Related questions
Question
Understanding the exceptional electron configurations in the first transition series.
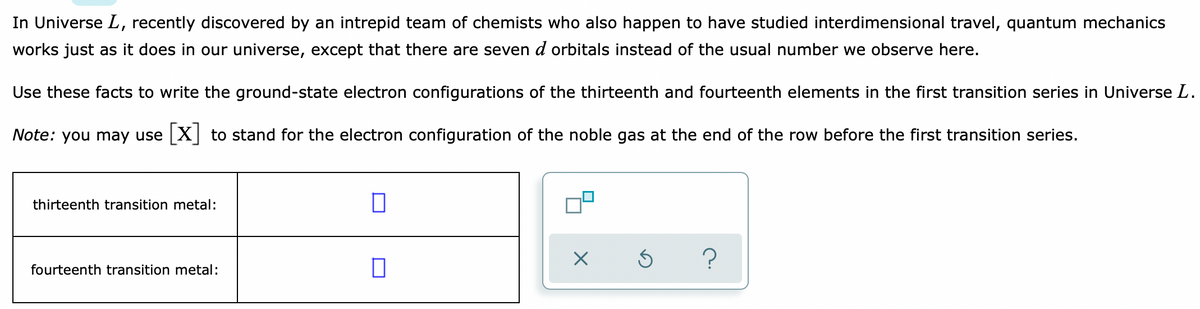
Transcribed Image Text:In Universe L, recently discovered by an intrepid team of chemists who also happen to have studied interdimensional travel, quantum mechanics
works just as it does in our universe, except that there are seven d orbitals instead of the usual number we observe here.
Use these facts to write the ground-state electron configurations of the thirteenth and fourteenth elements in the first transition series in Universe L.
Note: you may use X to stand for the electron configuration of the noble gas at the end of the row before the first transition series.
thirteenth transition metal:
fourteenth transition metal:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co