When comparing the first ionization energies of elements within the same period, it is noted that boron first ionization energy than berylium. This is due to the| greater shielding has a of the electrons from the subshell to an area closer to the nucleus, which is not possible for boron electron present in the shielded. making it the atom. As a result, this electron is penetration to remove. 2p harder beryllium smaller ineffectively easier effectively 2s
When comparing the first ionization energies of elements within the same period, it is noted that boron first ionization energy than berylium. This is due to the| greater shielding has a of the electrons from the subshell to an area closer to the nucleus, which is not possible for boron electron present in the shielded. making it the atom. As a result, this electron is penetration to remove. 2p harder beryllium smaller ineffectively easier effectively 2s
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 69SCQ: Answer the following questions about first ionization energies. (a) Generally ionization energies...
Related questions
Question
11ab please answer them all
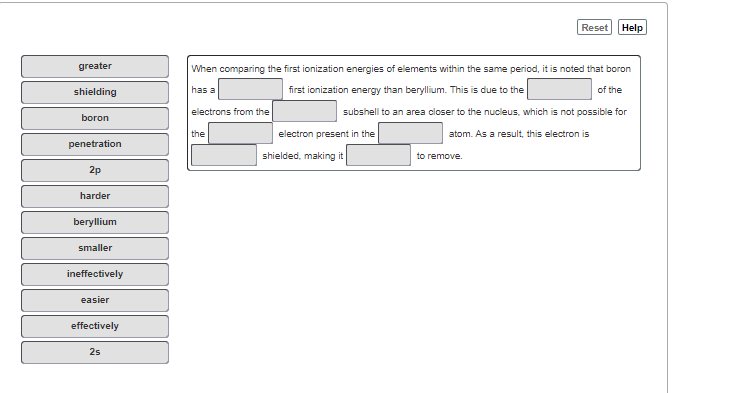
Transcribed Image Text:Reset Help
greater
When comparing the first ionization energies of elements within the same period, it is noted that boron
shielding
has a
first ionization energy than beryllium. This is due to the
of the
electrons from the
subshell to an area closer to the nucleus, which is not possible for
boron
the
electron present in the
atom. As a result, this electron is
penetration
shielded, making it
to remove.
2p
harder
beryllium
smaller
ineffectively
easier
effectively
2s
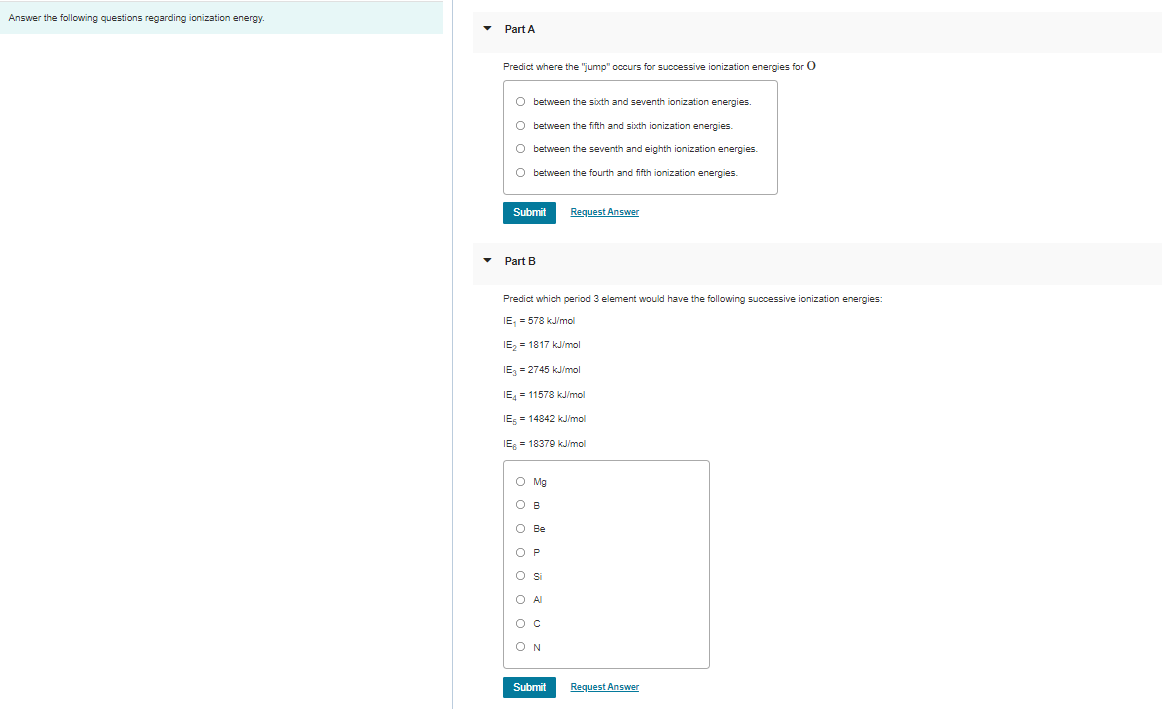
Transcribed Image Text:Answer the following questions regarding ionization energy.
Part A
Predict where the "jump" occurs for successive ionization energies for O
O between the sixth and seventh ionization energies.
O between the fifth and sixth ionization energies
O between the seventh and eighth ionization energies.
O between the fourth and fifth ionization energies.
Submit
Request Answer
Part B
Predict which period 3 element would have the following successive ionization energies:
IE, = 578 kJ/mol
IE, = 1817 kJ/mol
IE, = 2745 kJ/mol
IE = 11578 kJ/mol
IES = 14842 kJ/mol
IE = 18379 kJ/mol
O Mg
O B
O Be
OP
O Al
ON
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
