In which reaction is water acting only as a base? NH3(aq) + H2O(1) --> NH4* + OH HNO3(aq) + H2O(1) --> H3O* + NO3 2 H20(1) --> H3O* + OH¯ 2 H2O2(aq) --> 2 H20(1) + O2(g)
In which reaction is water acting only as a base? NH3(aq) + H2O(1) --> NH4* + OH HNO3(aq) + H2O(1) --> H3O* + NO3 2 H20(1) --> H3O* + OH¯ 2 H2O2(aq) --> 2 H20(1) + O2(g)
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.15E: The following reactions illustrate Brnsted acid-base behavior. Complete each equation....
Related questions
Question
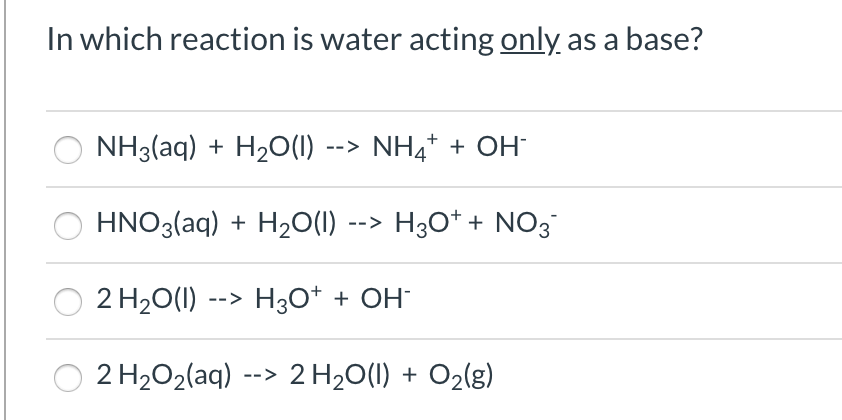
Transcribed Image Text:In which reaction is water acting only as a base?
NH3(aq) + H2O(1) --> NH4* + OH
HNO3(aq) + H2O(1) --> H3O* + NO3
2 H20(1) --> H3O* + OH¯
2 H2O2(aq) --> 2 H20(1) + O2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning