Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. For many purposes we can treat propane (C,H,) as an ideal gas at temperatures above its boling point of -42. "C. Suppose the temperature of a sample of propane gas is lowered from 24.0 °C to -14.0 C, and at the same time the pressure is changed. If the initial pressure was 0.52 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to the correct number of significant digits. 0.82 kPa
Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. For many purposes we can treat propane (C,H,) as an ideal gas at temperatures above its boling point of -42. "C. Suppose the temperature of a sample of propane gas is lowered from 24.0 °C to -14.0 C, and at the same time the pressure is changed. If the initial pressure was 0.52 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to the correct number of significant digits. 0.82 kPa
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 98QAP: Dinitrogen monoxide, N2O, reacts with propane, C3H8, to farm nitrogen, N2; carbon dioxide. CO2; and...
Related questions
Question
Please hand written solution only
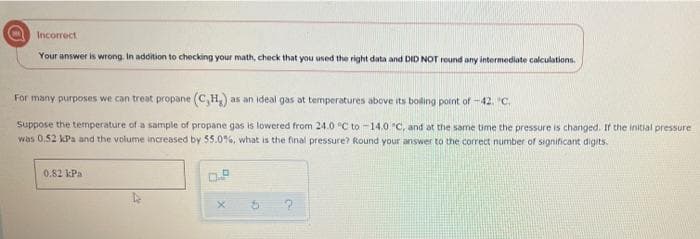
Transcribed Image Text:Incorrect
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
For many purposes we can treat propane (C,H,) as an ideal gas at temperatures above its boling pornt of -42. "c,
Suppose the temperature of a sample of propane gas is lowered from 24.0 °C to -14.0 C, and at the same time the pressure is changed. If the initial pressure
was 0.52 kPa and the volume increased by 55.0%, what is the final pressure? Round your answer to the correct number of significant digits.
0.82 kPa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning