Int molecular forces of attraction are often important in holding large molecules together. For example, some proteins fold into compact shapes, held together by attractive forces between nearby functional groups. A schematic of a folded protein is shown below, with the protein backbone indicated by a blue-green ribbon, and various appendages drawn dangling from the chain. What type of intramolecular force occurs at site B? D E CH, CHO HOCH, - CH,C C=0-------- H-N C hydrogen bond (CH;l,NH, CH, CH,CH(CHla CH CH, CH,-S S-CH, hydrogen bond CH, CHCH CH helical structure -CH, disulfide bond F van der Waals forces dipole-dipole interactions hydrogen bonding ion-ion interactions O O O O
Int molecular forces of attraction are often important in holding large molecules together. For example, some proteins fold into compact shapes, held together by attractive forces between nearby functional groups. A schematic of a folded protein is shown below, with the protein backbone indicated by a blue-green ribbon, and various appendages drawn dangling from the chain. What type of intramolecular force occurs at site B? D E CH, CHO HOCH, - CH,C C=0-------- H-N C hydrogen bond (CH;l,NH, CH, CH,CH(CHla CH CH, CH,-S S-CH, hydrogen bond CH, CHCH CH helical structure -CH, disulfide bond F van der Waals forces dipole-dipole interactions hydrogen bonding ion-ion interactions O O O O
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter21: Biochemistry
Section: Chapter Questions
Problem 8QAP
Related questions
Question
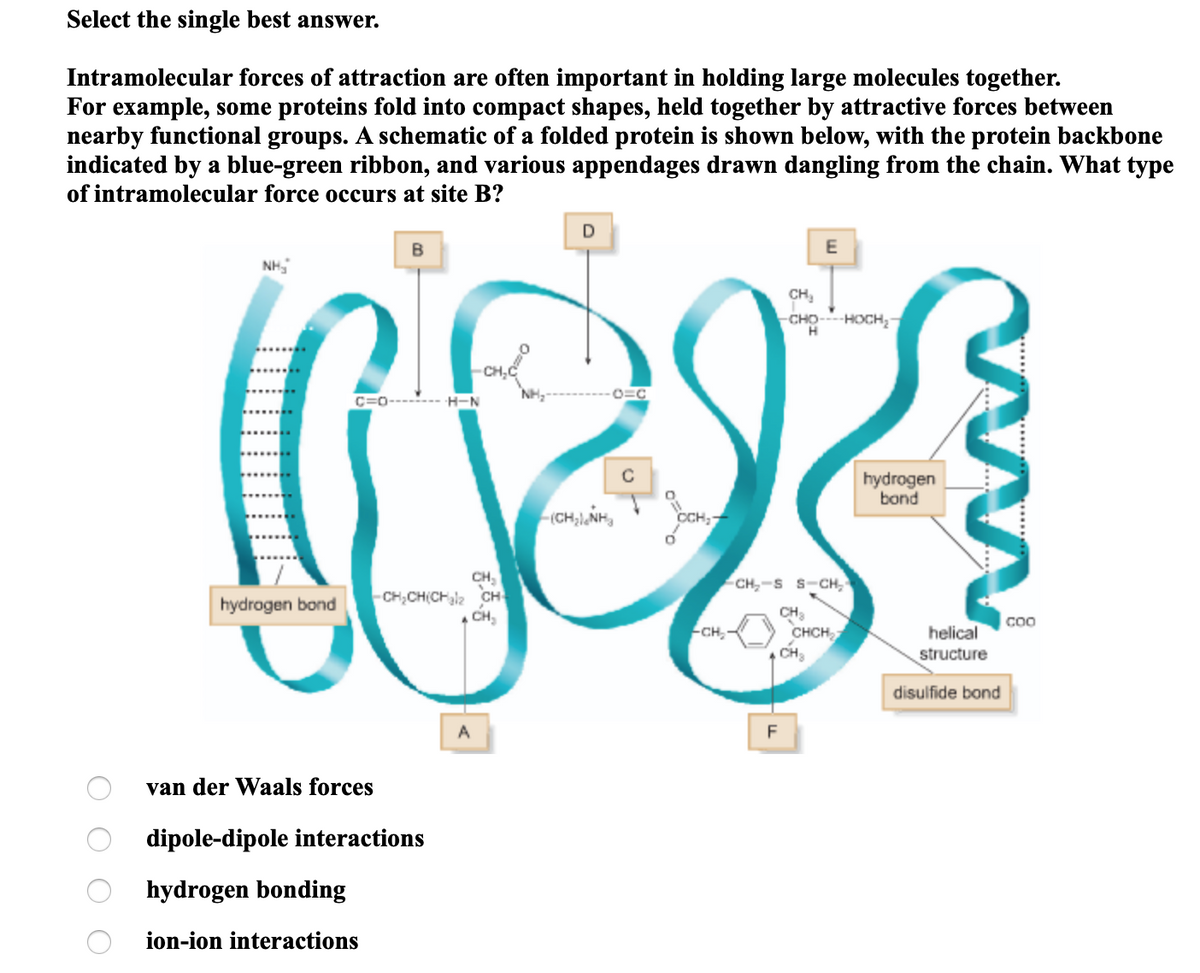
Transcribed Image Text:Select the single best answer.
Intramolecular forces of attraction are often important in holding large molecules together.
For example, some proteins fold into compact shapes, held together by attractive forces between
nearby functional groups. A schematic of a folded protein is shown below, with the protein backbone
indicated by a blue-green ribbon, and various appendages drawn dangling from the chain. What type
of intramolecular force occurs at site B?
E
NH,
CH,
-HOCH;-
O=C
H-N
hydrogen
bond
CCH,
CH,
CH,-S S-CH,
hydrogen bond
-CH,CH(CHl2 CH
CH,
CH
CHCH
-CH,
helical
structure
| disulfide bond
van der Waals forces
dipole-dipole interactions
hydrogen bonding
ion-ion interactions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning