Iodine is sparingly soluble in water but much more so in carbon tetrachloride. The equilibrium constant, also called the partition coefficient, for the distribution of I, between these two phases I2(aq) =12(CCI4) is 83 at 20°C. (a) A student adds 0.028 L of CCI4 to 0.213 L of an aqueous solution containing 0.037 g Iz. The mixture is shaken and the two phases are then allowed to separate. Calculate the fraction of I2 remaining in the aqueous phase. 49) 8.396 x (b) The student now repeats the extraction of I2 with another 0.028 L 40.0002608 Cl4. Calculate the fraction of I2 from the original solution that remains in the aqueous phase. (c) Compare the result in (b) with a single extraction using 0.056 L of CCI4. Comment on the difference. After a single extraction using 0.056 L of CC4, the fraction of I, that remains in the aqueous phase is 49 4.3 x . This result is larger Vy than the result in (b), which means that a single large extraction is not as efficient as vy two separate smaller extractions.
Iodine is sparingly soluble in water but much more so in carbon tetrachloride. The equilibrium constant, also called the partition coefficient, for the distribution of I, between these two phases I2(aq) =12(CCI4) is 83 at 20°C. (a) A student adds 0.028 L of CCI4 to 0.213 L of an aqueous solution containing 0.037 g Iz. The mixture is shaken and the two phases are then allowed to separate. Calculate the fraction of I2 remaining in the aqueous phase. 49) 8.396 x (b) The student now repeats the extraction of I2 with another 0.028 L 40.0002608 Cl4. Calculate the fraction of I2 from the original solution that remains in the aqueous phase. (c) Compare the result in (b) with a single extraction using 0.056 L of CCI4. Comment on the difference. After a single extraction using 0.056 L of CC4, the fraction of I, that remains in the aqueous phase is 49 4.3 x . This result is larger Vy than the result in (b), which means that a single large extraction is not as efficient as vy two separate smaller extractions.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
100%
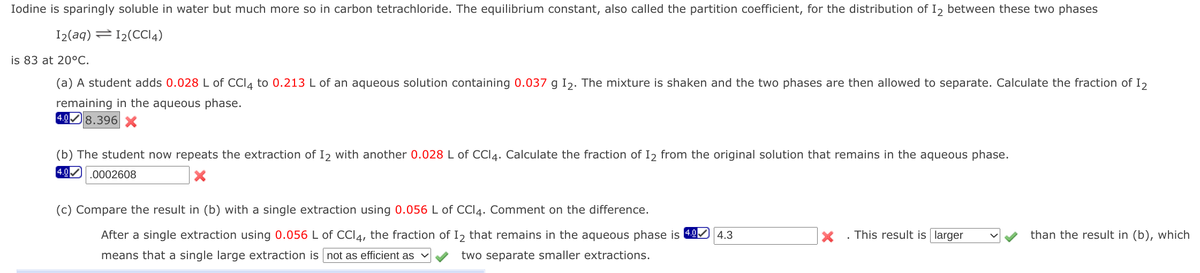
Transcribed Image Text:Iodine is sparingly soluble in water but much more so in carbon tetrachloride. The equilibrium constant, also called the partition coefficient, for the distribution of I2 between these two phases
I2(aq) =12(CCI4)
is 83 at 20°C.
(a) A student adds 0.028 L of CCI4 to 0.213 L of an aqueous solution containing 0.037 g Iɔ. The mixture is shaken and the two phases are then allowed to separate. Calculate the fraction of I,
remaining in the aqueous phase.
4.08.396 X
(b) The student now repeats the extraction of I2 with another 0.028 L of CCI4. Calculate the fraction of I2 from the original solution that remains in the aqueous phase.
4.0 .0002608
(c) Compare the result in (b) with a single extraction using 0.056 L of CCI4. Comment on the difference.
After a single extraction using 0.056 L of CCI4, the fraction of I2 that remains in the aqueous phase is 49
4.3
This result is larger
than the result in (b), which
means that a single large extraction is not as efficient as
two separate smaller extractions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,