ion. In this reaction, glutamate is converted to an a-keto acid. he structure to show the a-keto acid product. II I CH₂ I -C CH₂ enzyme + NAD+ + H₂O. I =C₂ Incorrect chemical formulas for the remaining products. g products: NADH + H++ NH he enzyme that catalyzes the reaction. CH₂ I CH₂ OH OH gn + remaining produ
ion. In this reaction, glutamate is converted to an a-keto acid. he structure to show the a-keto acid product. II I CH₂ I -C CH₂ enzyme + NAD+ + H₂O. I =C₂ Incorrect chemical formulas for the remaining products. g products: NADH + H++ NH he enzyme that catalyzes the reaction. CH₂ I CH₂ OH OH gn + remaining produ
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 60AP
Related questions
Question
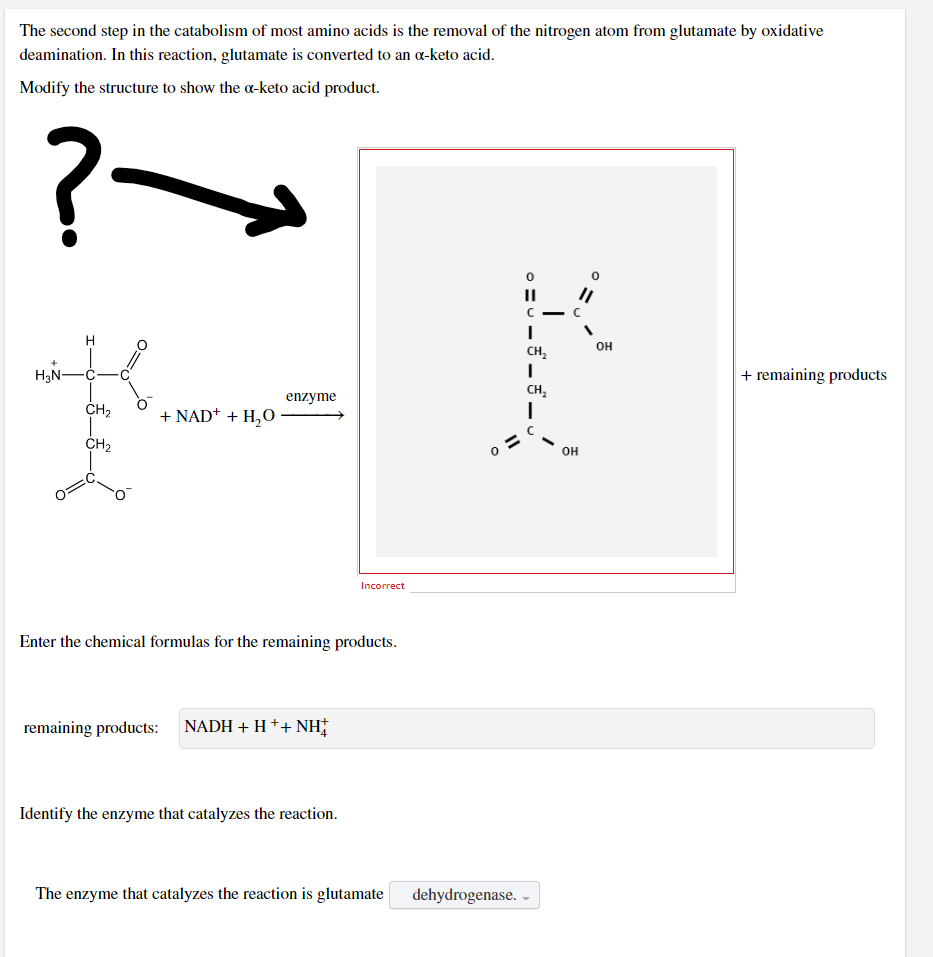
Transcribed Image Text:The second step in the catabolism of most amino acids is the removal of the nitrogen atom from glutamate by oxidative
deamination. In this reaction, glutamate is converted to an a-keto acid.
Modify the structure to show the a-keto acid product.
?-
||
с
I
CH₂
I
H₂N-
&
+ remaining products
enzyme
CH₂
I
CH₂
+ NAD+ + H₂O
CH₂
Incorrect
Enter the chemical formulas for the remaining products.
remaining products: NADH + H+ + NH
Identify the enzyme that catalyzes the reaction.
The enzyme that catalyzes the reaction is glutamate
dehydrogenase.
OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning