Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patient's body to treat cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lowering the risk of damage to healthy tissue. Iridium-192 is often used in the head or breast. Use the radioactive decay curve of iridium-192 to answer the three questions. 100 90 - 80 70 - 60 - 50 - 40 - 30 - 20 - 10- 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 Time (days) If the initial sample is 4.50 g, what mass of the original iridium-192 remains after 35 days? Sample remaining (%)
Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patient's body to treat cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lowering the risk of damage to healthy tissue. Iridium-192 is often used in the head or breast. Use the radioactive decay curve of iridium-192 to answer the three questions. 100 90 - 80 70 - 60 - 50 - 40 - 30 - 20 - 10- 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 Time (days) If the initial sample is 4.50 g, what mass of the original iridium-192 remains after 35 days? Sample remaining (%)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.36PAE
Related questions
Question

Transcribed Image Text:If the initial sample is 4.50 g, what mass of the original iridium-192 remains after 35 days?
mass remaining:
Estimate the half-life of the radioisotope.
half-life:
days
How many days would it take for two-thirds of the sample to decay?
time:
days
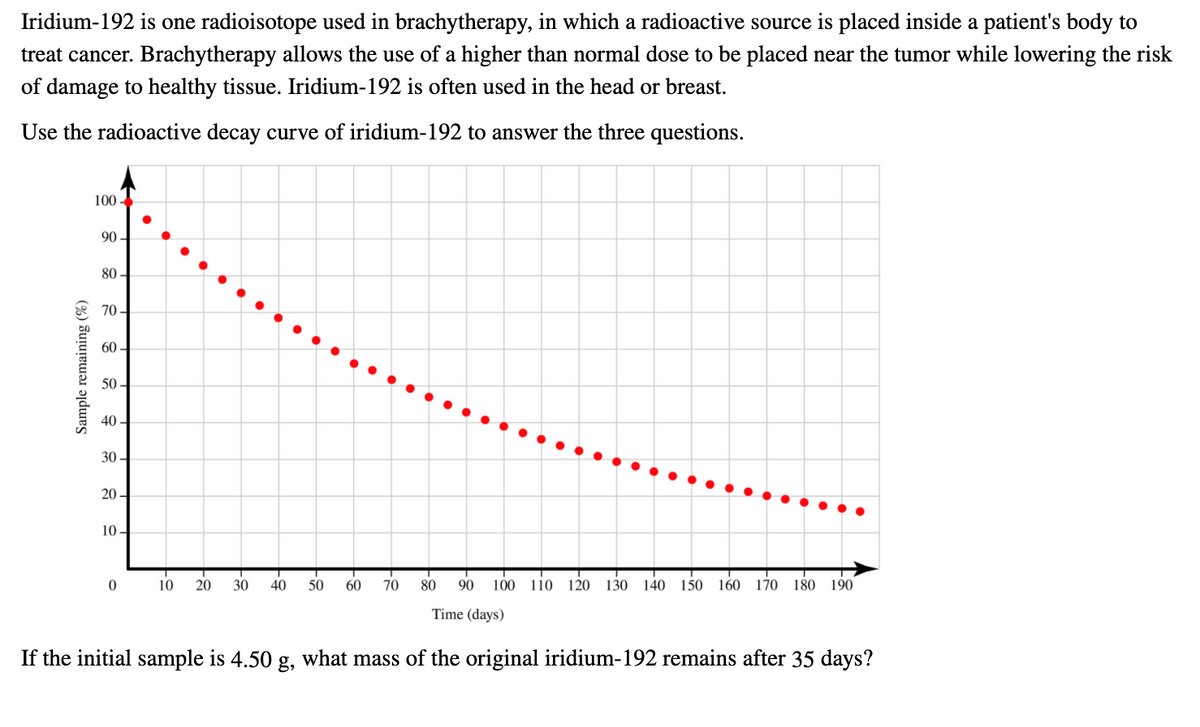
Transcribed Image Text:Iridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a patient's body to
treat cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lowering the risk
of damage to healthy tissue. Iridium-192 is often used in the head or breast.
Use the radioactive decay curve of iridium-192 to answer the three questions.
100-
90 -
80 –
70 -
60 -
50 -
40 -
30 -
20 -
10 -
10
20
30
40
50
60
70
80
90
100 110 120 130 140
150 160
170
180 190
Time (days)
If the initial sample is 4.50 g, what mass of the original iridium-192 remains after 35 days?
Sample remaining (%)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER