Iron(II) ion is oxidized by chlorine in aqueous solution, the overall equation being 2 Fe2+ + Cl2 →2 Fe³* + 2 Cl- It is found experimentally that the rate of the overall reac- tion is decreased when either the iron(III) ion or the chloride-ion concentration is increased. Which of the fol- lowing possible mechanisms is consistent with the experi- mental observations? (a) (1) Fe²* + Cl, 2 Fe* + Cl¯ + Cl k-1 (rapid equilibrium) (2) Fe²+ + CI ka Fe+ + CI" (negligible reverse rate) ks (b) (3) Fe²* + Cl2 - Fe(IV) + 2 CI¯ (rapid equilibrium) (4) Fe(IV) + Fe²+ 2 Fe+ (negligible reverse rate) where Fe(IV) is Fe in the (+IV) oxidation state.
Iron(II) ion is oxidized by chlorine in aqueous solution, the overall equation being 2 Fe2+ + Cl2 →2 Fe³* + 2 Cl- It is found experimentally that the rate of the overall reac- tion is decreased when either the iron(III) ion or the chloride-ion concentration is increased. Which of the fol- lowing possible mechanisms is consistent with the experi- mental observations? (a) (1) Fe²* + Cl, 2 Fe* + Cl¯ + Cl k-1 (rapid equilibrium) (2) Fe²+ + CI ka Fe+ + CI" (negligible reverse rate) ks (b) (3) Fe²* + Cl2 - Fe(IV) + 2 CI¯ (rapid equilibrium) (4) Fe(IV) + Fe²+ 2 Fe+ (negligible reverse rate) where Fe(IV) is Fe in the (+IV) oxidation state.
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 44E: The rate of the reaction O(g)+NO2(g)NO(g)+O2(g) was studied at a certain temperature. a. In one...
Related questions
Question
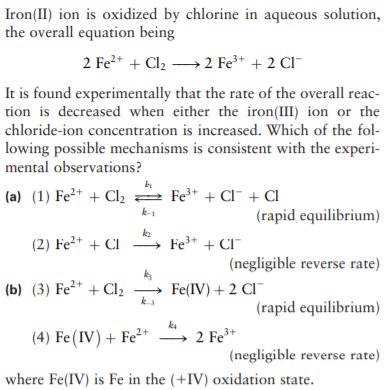
Transcribed Image Text:Iron(II) ion is oxidized by chlorine in aqueous solution,
the overall equation being
2 Fe2+ + Cl2 →2 Fe³* + 2 Cl-
It is found experimentally that the rate of the overall reac-
tion is decreased when either the iron(III) ion or the
chloride-ion concentration is increased. Which of the fol-
lowing possible mechanisms is consistent with the experi-
mental observations?
(a) (1) Fe²* + Cl, 2 Fe* + Cl¯ + Cl
k-1
(rapid equilibrium)
(2) Fe²+ + CI
ka
Fe+ + CI"
(negligible reverse rate)
ks
(b) (3) Fe²* + Cl2
-
Fe(IV) + 2 CI¯
(rapid equilibrium)
(4) Fe(IV) + Fe²+
2 Fe+
(negligible reverse rate)
where Fe(IV) is Fe in the (+IV) oxidation state.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning