Is this a reasonable structure? If not, why not? proposed Lewis molecule structure Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. : Br The correct number is: I Br, Br : No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. :F: No, the total number of valence electrons is wrong. BF 3 The correct number is: O :F B F: No, some atoms have the wrong number of electrons around them. .. The symbols of the problem atoms are: Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical :0: :- : : 0 :0:
Is this a reasonable structure? If not, why not? proposed Lewis molecule structure Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. : Br The correct number is: I Br, Br : No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. :F: No, the total number of valence electrons is wrong. BF 3 The correct number is: O :F B F: No, some atoms have the wrong number of electrons around them. .. The symbols of the problem atoms are: Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical :0: :- : : 0 :0:
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter1: Bond Angles And Shape
Section: Chapter Questions
Problem 12CTQ: Consider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this...
Related questions
Question
100%
Please help me with my assignment, thank you please be clear for answer boxes
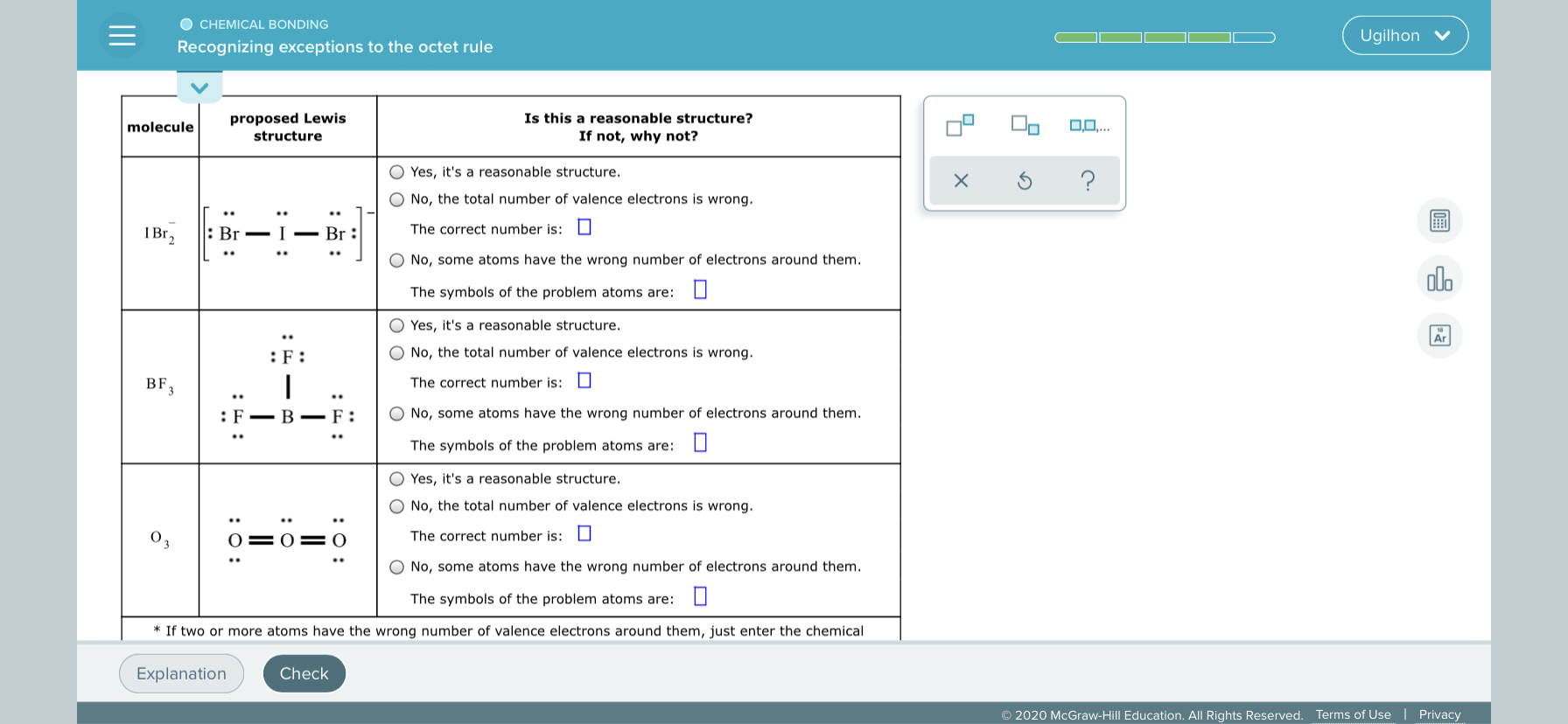
Transcribed Image Text:Is this a reasonable structure?
If not, why not?
proposed Lewis
molecule
structure
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
: Br
The correct number is:
I Br,
Br :
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
Yes, it's a reasonable structure.
:F:
No, the total number of valence electrons is wrong.
BF 3
The correct number is: O
:F
B
F:
No, some atoms have the wrong number of electrons around them.
..
The symbols of the problem atoms are:
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
The correct number is:
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
* If two or more atoms have the wrong number of valence electrons around them, just enter the chemical
:0:
:- :
: 0
:0:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning