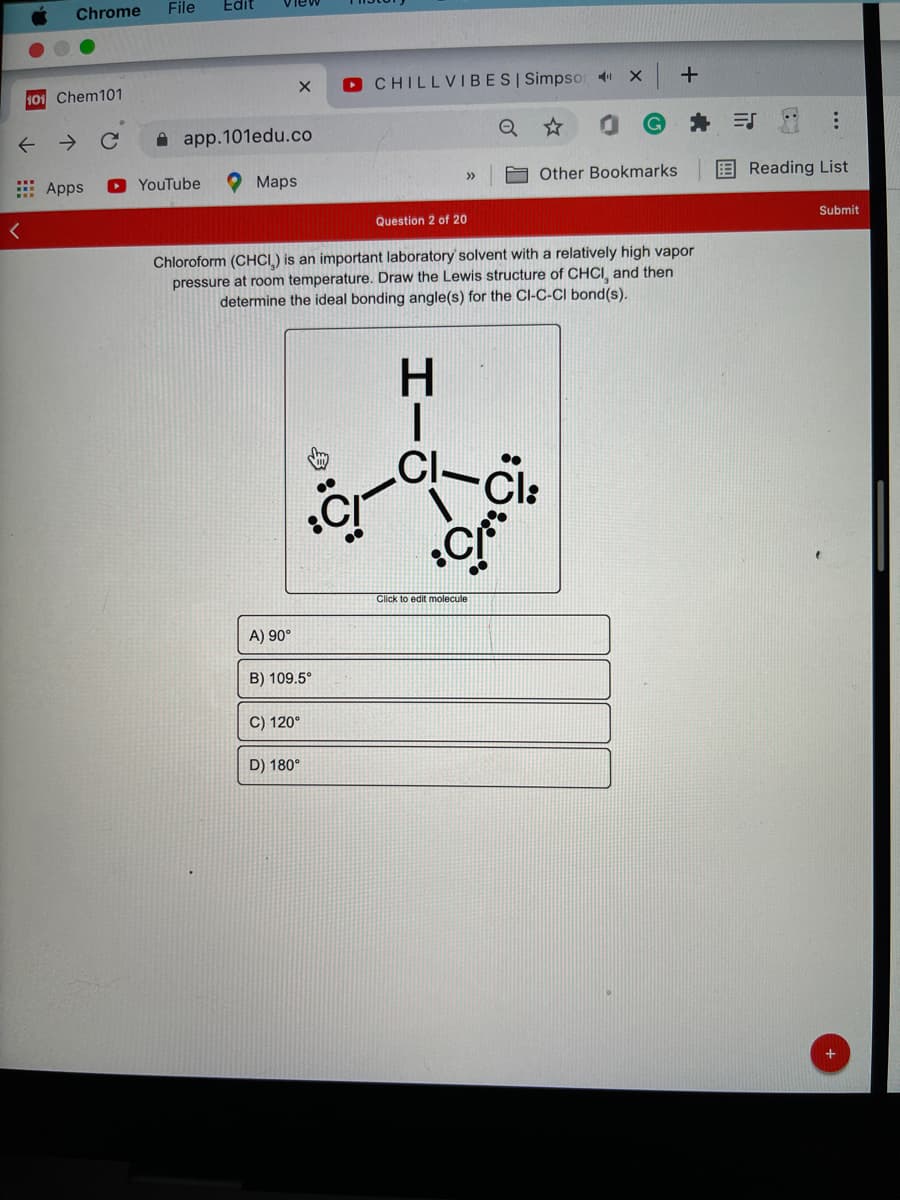
Chem101 O CHILLVIBESI Simpso 4 -> A app.101edu.co Apps O YouTube Maps A Other Bookmarks E Reading List >> Question 2 of 20 Submit Chloroform (CHCI) is an important laboratory' solvent with a relatively high vapor pressure at room temperature. Draw the Lewis structure of CHCI, and then determine the ideal bonding angle(s) for the CI-C-CI bond(s). Click to edit molecule A) 90° B) 109.5° C) 120° D) 180° HII
Chem101 O CHILLVIBESI Simpso 4 -> A app.101edu.co Apps O YouTube Maps A Other Bookmarks E Reading List >> Question 2 of 20 Submit Chloroform (CHCI) is an important laboratory' solvent with a relatively high vapor pressure at room temperature. Draw the Lewis structure of CHCI, and then determine the ideal bonding angle(s) for the CI-C-CI bond(s). Click to edit molecule A) 90° B) 109.5° C) 120° D) 180° HII
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 101AP: (a) Predict the geometry of the SbCl52 ion, using the VSEPR method. (b) The ion SbCl63 is prepared...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View
O CHILLVIBESI Simpsor
101 Chem101
->
A app.101edu.co
: Apps
O YouTube
O Maps
Other Bookmarks
E Reading List
>>
Question 2 of 20
Submit
Chloroform (CHCI.) is an important laboratory solvent with a relatively high vapor
pressure at room temperature. Draw the Lewis structure of CHCI, and then
determine the ideal bonding angle(s) for the ClI-C-CI bond(s).
H.
Cl:
Click to edit molecule
A) 90°
B) 109.5°
C) 120°
D) 180°
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning