It is found that up to 0.0110 g of SRF2 dissolves in 100 mL of aqueous solution at a certain temperature. Determine the value of Ksp for SRF2. 1 NEXT Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. SFF2(s) Sr* (aq) 2 F (aq) + Initial (M) Change (M) x+ +2x Equilibrium (M) +2x +x Incorrect, 3 attempts remaining Your Change in concentration for Sr2* is incorrect. In this problem, you should use the given molar solubility to determine the concentration of each ion at Equilibrium and hence the Change. Remember that the concentration of each ion will be directly proportional to the number of moles of the ion produced from 1 mole of the solid! Your Change in concentration for F is incorrect. In this problem, you should use the given molar solubility to determine the concentration of each ion at Equilibrium and hence the Change. Remember that the concentration of each ion will be directly proportional to the number of moles of the ion produced from 1 mole of the solid!
It is found that up to 0.0110 g of SRF2 dissolves in 100 mL of aqueous solution at a certain temperature. Determine the value of Ksp for SRF2. 1 NEXT Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. SFF2(s) Sr* (aq) 2 F (aq) + Initial (M) Change (M) x+ +2x Equilibrium (M) +2x +x Incorrect, 3 attempts remaining Your Change in concentration for Sr2* is incorrect. In this problem, you should use the given molar solubility to determine the concentration of each ion at Equilibrium and hence the Change. Remember that the concentration of each ion will be directly proportional to the number of moles of the ion produced from 1 mole of the solid! Your Change in concentration for F is incorrect. In this problem, you should use the given molar solubility to determine the concentration of each ion at Equilibrium and hence the Change. Remember that the concentration of each ion will be directly proportional to the number of moles of the ion produced from 1 mole of the solid!
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 113QRT
Related questions
Question
Please help me fix the ICE table and Ksp.
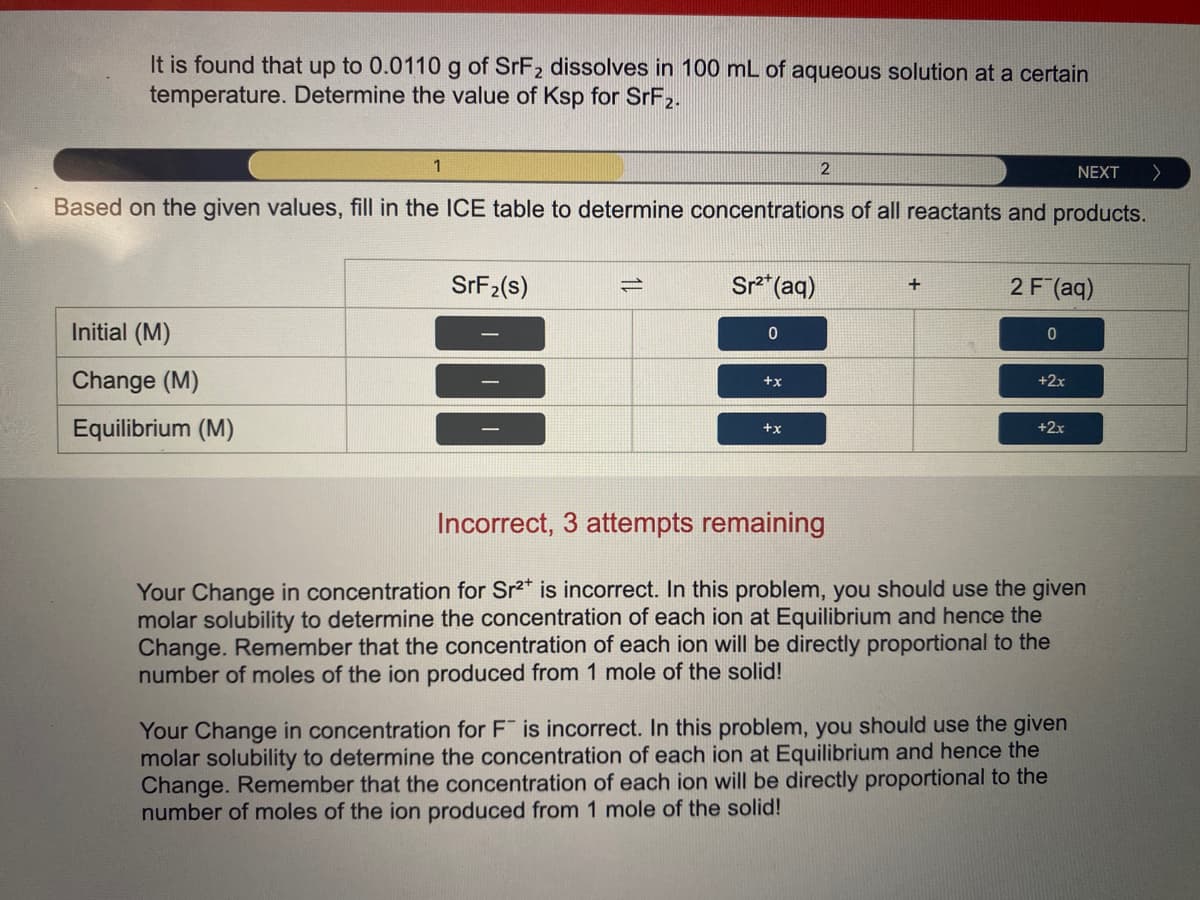
Transcribed Image Text:It is found that up to 0.0110 g of SrF2 dissolves in 100 mL of aqueous solution at a certain
temperature. Determine the value of Ksp for SRF2.
1
NEXT
へ
Based on the given values, fill in the ICE table to determine concentrations of all reactants and products.
SRF2(s)
Sr*(aq)
2 F (aq)
Initial (M)
Change (M)
+2x
+x
Equilibrium (M)
+2x
+x
Incorrect, 3 attempts remaining
Your Change in concentration for Sr2" is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!
Your Change in concentration for F is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!
![It is found that up to 0.0110 g of SRF2 dissolves in 100 mL of aqueous solution at a certain
temperature. Determine the value of Ksp for SrF2.
1
PREV
Based on the set up of your ICE table, construct the expression for Ksp and then evaluate it. Do not
combine or simplify terms.
2.68 x 10-9
Ksp
[8.76 x 10-1| [1.75 × 10**]²
%3D
Incorrect, 3 attempts remaining
Your Change in concentration for Sr2* is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!
Your Change in concentration for F is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Faeef920a-b7fc-42c4-a405-16f92aebf3b1%2F3a253dd7-4352-414e-8964-83c58230275c%2Fvngvfa5_processed.jpeg&w=3840&q=75)
Transcribed Image Text:It is found that up to 0.0110 g of SRF2 dissolves in 100 mL of aqueous solution at a certain
temperature. Determine the value of Ksp for SrF2.
1
PREV
Based on the set up of your ICE table, construct the expression for Ksp and then evaluate it. Do not
combine or simplify terms.
2.68 x 10-9
Ksp
[8.76 x 10-1| [1.75 × 10**]²
%3D
Incorrect, 3 attempts remaining
Your Change in concentration for Sr2* is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!
Your Change in concentration for F is incorrect. In this problem, you should use the given
molar solubility to determine the concentration of each ion at Equilibrium and hence the
Change. Remember that the concentration of each ion will be directly proportional to the
number of moles of the ion produced from 1 mole of the solid!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
