wing read 2H,S(9)+30,(9) 2 SO,(g)+2H,0(9) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.050. The engineer charges ("fills") four reaction vessels with hydrogen sulfide and oxygen, and lets the reaction begin, She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel expected change in pressure compound pressure H,S 7.18 atm B increase OI decrease O (no change) 149 atm O 1 increase OI decrease O (no change) so, $.37 atm O 1 increase OI decrease O (no change) H,0 7.40 atm O T Increase O I decrease O (no change) H,S 9.04 atm O 1 increase OI decrease O (no change) 10 27 atm O t increase OI decrease O (no change) so, 6 51 atm O 1 increase O I decrease O (no change) H,0 5.54 atm O t increase O I decrease O (no change) H,S 8.11 atm O T Increase OI decrease O (no change) S. S8 atm O 1 increase Ol decrease O (no change) so, 7.44 atm O T increase OI decrease O (no change) H,0 6 47 atm O T increase OI decrease O (no change) |目 目
wing read 2H,S(9)+30,(9) 2 SO,(g)+2H,0(9) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.050. The engineer charges ("fills") four reaction vessels with hydrogen sulfide and oxygen, and lets the reaction begin, She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel expected change in pressure compound pressure H,S 7.18 atm B increase OI decrease O (no change) 149 atm O 1 increase OI decrease O (no change) so, $.37 atm O 1 increase OI decrease O (no change) H,0 7.40 atm O T Increase O I decrease O (no change) H,S 9.04 atm O 1 increase OI decrease O (no change) 10 27 atm O t increase OI decrease O (no change) so, 6 51 atm O 1 increase O I decrease O (no change) H,0 5.54 atm O t increase O I decrease O (no change) H,S 8.11 atm O T Increase OI decrease O (no change) S. S8 atm O 1 increase Ol decrease O (no change) so, 7.44 atm O T increase OI decrease O (no change) H,0 6 47 atm O T increase OI decrease O (no change) |目 目
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.101PAE: 12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to...
Related questions
Question
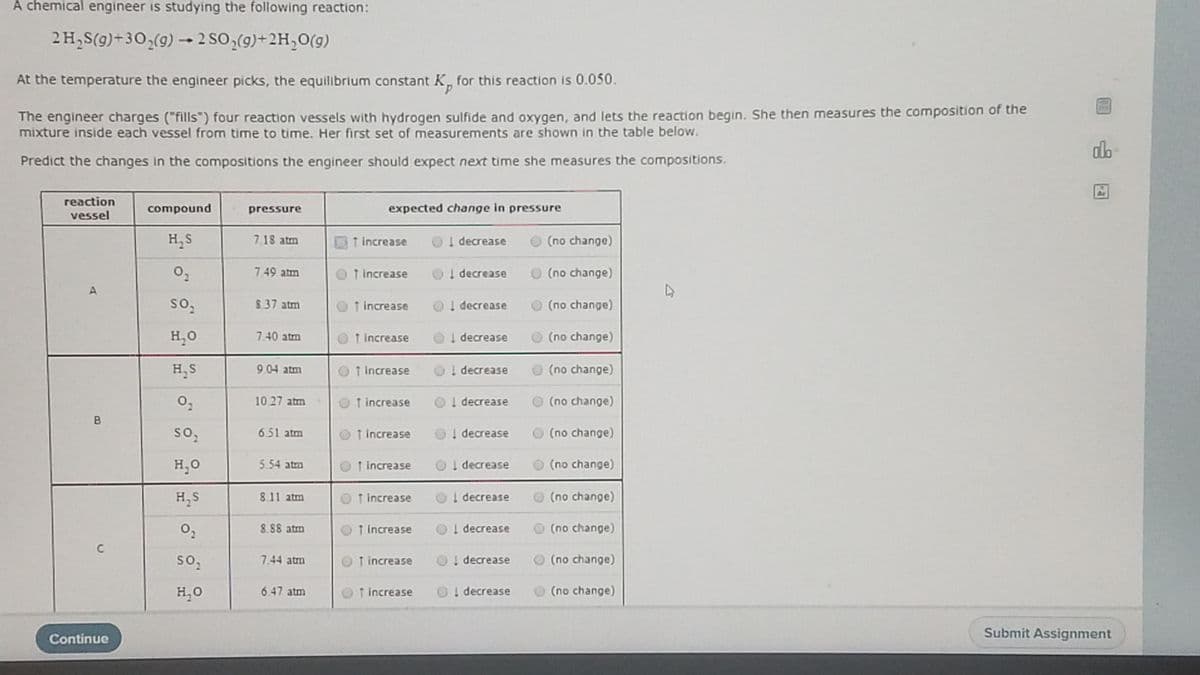
Transcribed Image Text:A chemical engineer is studying the following reaction:
2 H,S(g)+3O,(9) 2 SO,(9)+2H,0(g)
At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.050.
The engineer charges ("fills") four reaction vessels with hydrogen sulfide and oxygen, and lets the reaction begin. She then measures the composition of the
mixture inside each vessel from time to time. Her first set of measurements are shown in the table below.
dlo
Predict the changes in the compositions the engineer should expect next time she measures the compositions.
Ar
reaction
compound
pressure
expected change in pressure
vessel
H,S
7.18 atm
↑ increase
O
I decrease
O (no change)
7.49 atm
O 1 increase
OI decrease
O (no change)
8.37 atm
↑ increase
O ! decrease
O (no change)
H,0
7.40 atm
OT Increase
O
I decrease
O (no change)
H,S
904 atm
O 1 Increase
Ol decrease
O (no change)
1027 atm
O t increase
OI decrease
O (no change)
so,
651 atm
1 increase
O
! decrease
O (no change)
H,0
5.54 atm
O t increase
OI decrease
O (no change)
H, S
811 atm
O
T increase
OI decrease
O(no change)
8.88 atm
OT Increase
Ol decrease
(no change)
C
7.44 atm
T increase
OI decrease
O (no change)
H,O
6.47 atm
↑ increase
O! decrease
(no change)
Submit Assignment
Continue
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning