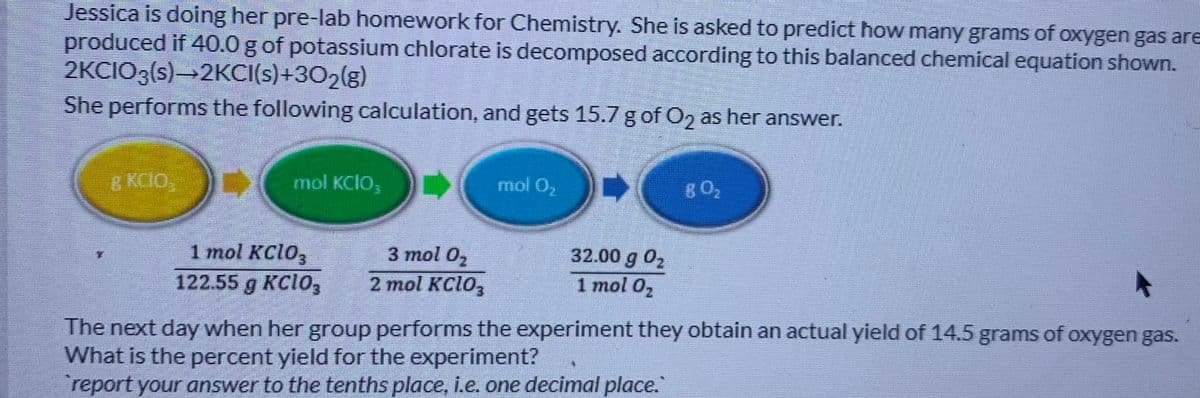
Jessica is doing her pre-lab homework for Chemistry. She is asked to predict how many grams of oxygen gas are produced if 40.0 g of potassium chlorate is decomposed according to this balanced chemical equation shown. 2KCIO3(s)→2KCI(s)+302(g) She performs the following calculation, and gets 15.7 g of O2 as her answer. & KCIO, mol KCIO, mol 02 802 32.00 g 02 1 mol 02 3 mol 02 1 mol KC103 122.55 g KC1O, 2 mol KC1O3 The next day when her group performs the experiment they obtain an actual yield of 14.5 grams of oxygen gas. What is the percent yield for the experiment? report your answer to the tenths place, i.e. one decimal place.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Step by step
Solved in 2 steps with 2 images









