JF Element information. Number 7 on top. Number 16 on the bottom. Draw a Bohr model indicating the number and location of the protons, neutrons, and electrons in one JF atom. Upload your clearly labeled drawing as your answer to this question. You may create your drawing by hand on paper or using your computer, but the drawing must be an original image created by you.
JF Element information. Number 7 on top. Number 16 on the bottom. Draw a Bohr model indicating the number and location of the protons, neutrons, and electrons in one JF atom. Upload your clearly labeled drawing as your answer to this question. You may create your drawing by hand on paper or using your computer, but the drawing must be an original image created by you.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter5: Atomic Theory : The Nuclear Model Of The Atom
Section: Chapter Questions
Problem 1CLE: Write a brief description of the relationships among each of the following groups of terms or...
Related questions
Question
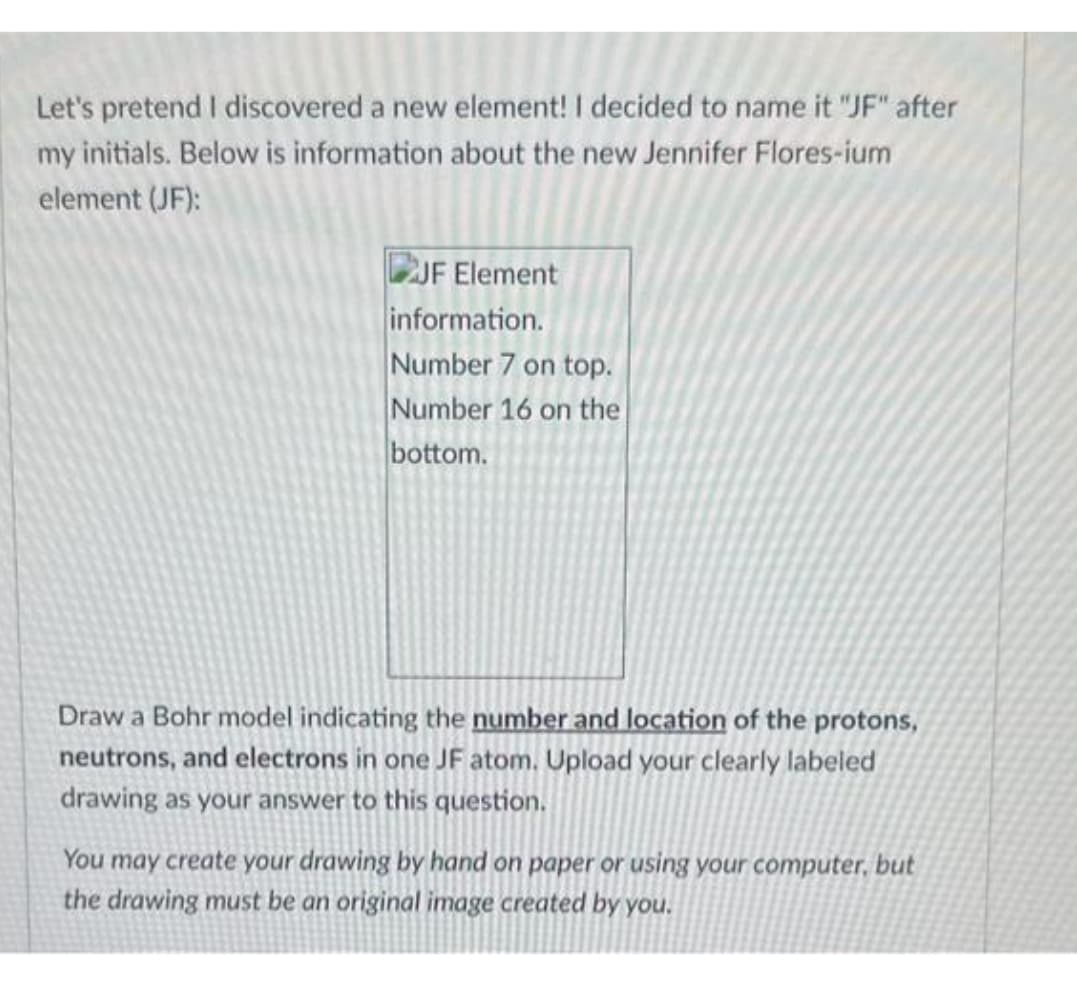
Transcribed Image Text:Let's pretend I discovered a new element! I decided to name it "JF" after
my initials. Below is information about the new Jennifer Flores-ium
element (JF):
JF Element
information.
Number 7 on top.
Number 16 on the
bottom.
Draw a Bohr model indicating the number and location of the protons,
neutrons, and electrons in one JF atom. Upload your clearly labeled
drawing as your answer to this question.
You may create your drawing by hand on paper or using your computer, but
the drawing must be an original image created by you.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning