Jo # valence Lewis e dot structure Electron Geometry Molecular Geometry Polar/ %23 Electron Sol puog Nonpolar/ Angle Main Atoms bonded Molecule #LP osu H IM Force groups H2S PH3 CCl4 CS2 SO2 O3
Jo # valence Lewis e dot structure Electron Geometry Molecular Geometry Polar/ %23 Electron Sol puog Nonpolar/ Angle Main Atoms bonded Molecule #LP osu H IM Force groups H2S PH3 CCl4 CS2 SO2 O3
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 7EQ: 7. Dimethyl ether
No. of electrons in structure _____
No. of valence electrons...
Related questions
Question
Experiment 6: PLEASE ANSWER ALL
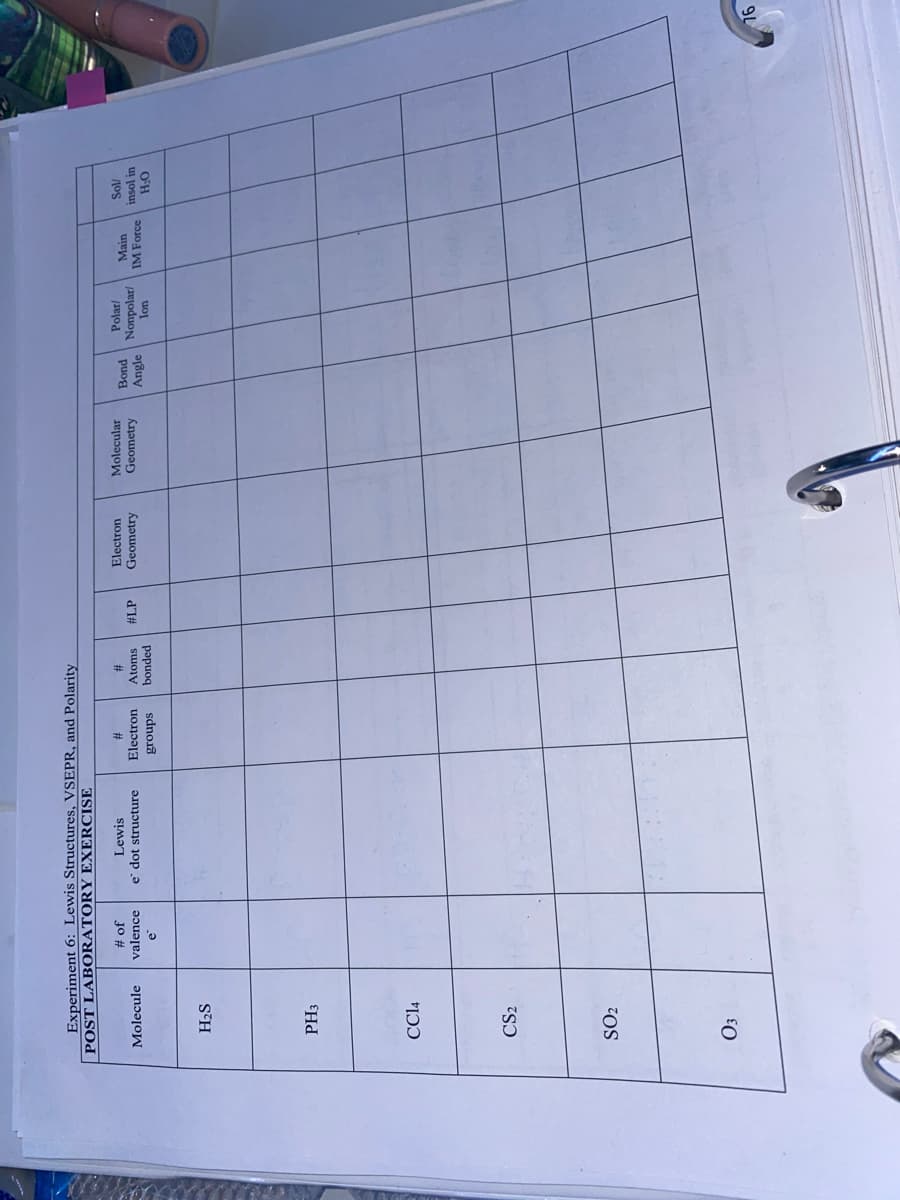
Transcribed Image Text:Experiment 6: Lewis Structures, VSEPR, and Polarity
POST LABORATORY EXERCISE
Jo #
valence
Lewis
Electron
Molecular
#
Electron
Polar/
2#
Atoms
Main
puog
Nonpolar/
Angle
Molecule
e' dot structure
#LP
Geometry
Geometry
IM Force
insol in
bonded
sdnor8
uo
OFH
H2S
PH3
CC14
CS2
SO2
Transcribed Image Text:10
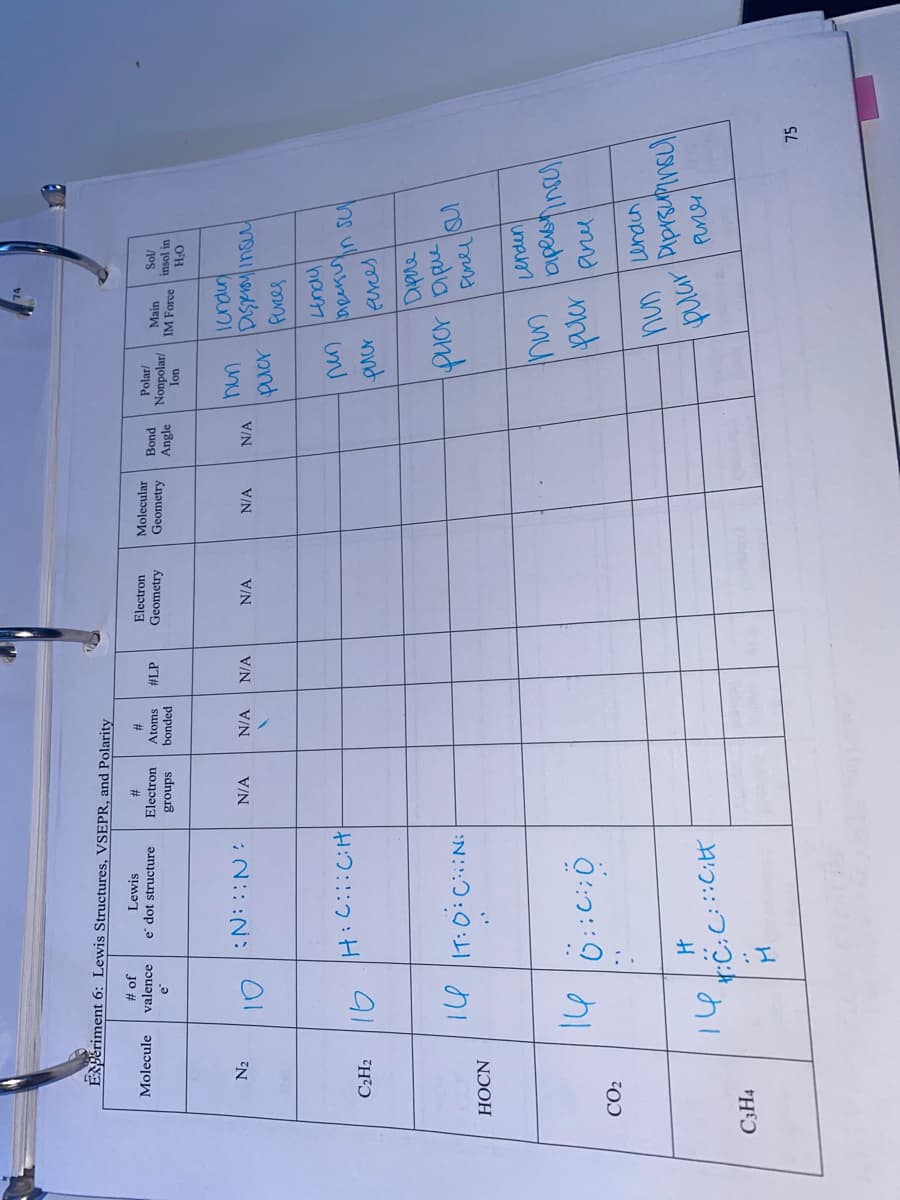
ENPěriment 6: Lewis Structures, VSEPR, and Polarity
Jo #
valence
e' dot structure
Lewis
2#
Atoms
Electron
Molecular
Geometry
Molecule
Polar/
Electron
#LP
Geometry
Nonpolar/
Main
puog
insol in
bonded
Angle
IM Force
sdno13
uo
O'H
N2
Kmpuni
N/A
N/A
N/A
N/A
N/A
N/A
:N:: ::
un
in sul
C2H2
puer leres
Fene S
HOCN
umpum
pur ene
CO2
Puner
H:C:C::i
C3H4
75
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER