JOP3JH-1J n/WsvrRAXK6XnHkiRvH2t180AR2-EPP O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0). What mass of water is produced by the reaction of 9.8 g of oxygen gas? Round your answer to 2 significant digits. x10 Explanation Check 2020 McC DELL -> %23 $ & 4 8.
JOP3JH-1J n/WsvrRAXK6XnHkiRvH2t180AR2-EPP O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0). What mass of water is produced by the reaction of 9.8 g of oxygen gas? Round your answer to 2 significant digits. x10 Explanation Check 2020 McC DELL -> %23 $ & 4 8.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 93CP: Consider the following unbalanced chemical equation for the combustion of pentane (C5H12):...
Related questions
Question
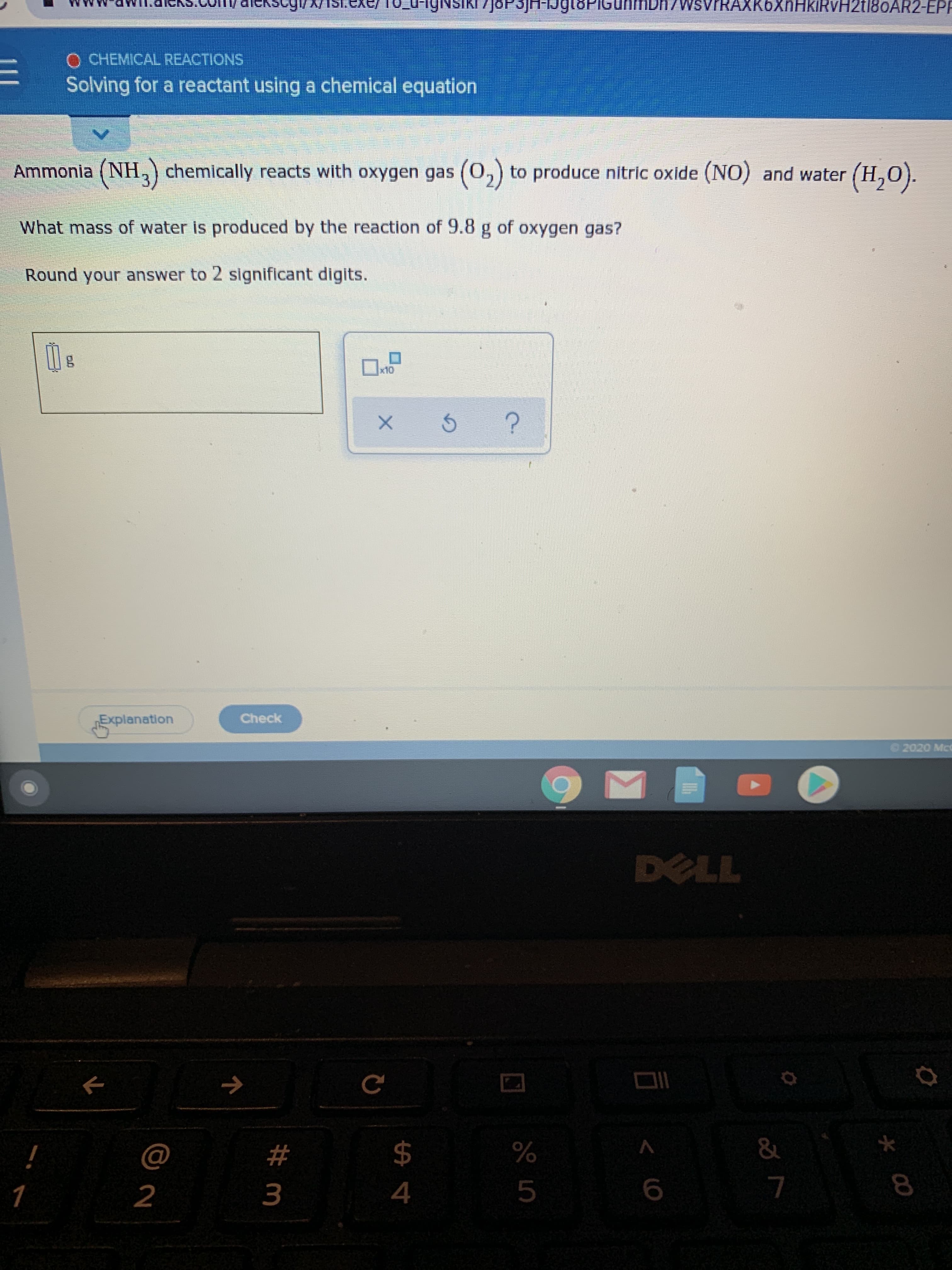
Transcribed Image Text:JOP3JH-1J
n/WsvrRAXK6XnHkiRvH2t180AR2-EPP
O CHEMICAL REACTIONS
Solving for a reactant using a chemical equation
Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0).
What mass of water is produced by the reaction of 9.8 g of oxygen gas?
Round your answer to 2 significant digits.
x10
Explanation
Check
2020 McC
DELL
->
%23
$
&
4
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning