L Calculate the mg/, Malorty for (ii) How much oxygen is required to oxide 100 mg/l of Glucose using the following reaction (balance the equation first) C6H1206 02 → CO2 + H20
L Calculate the mg/, Malorty for (ii) How much oxygen is required to oxide 100 mg/l of Glucose using the following reaction (balance the equation first) C6H1206 02 → CO2 + H20
Sustainable Energy
2nd Edition
ISBN:9781337551663
Author:DUNLAP, Richard A.
Publisher:DUNLAP, Richard A.
Chapter7: Energy From Nuclear Fusion
Section: Chapter Questions
Problem 14P
Related questions
Question
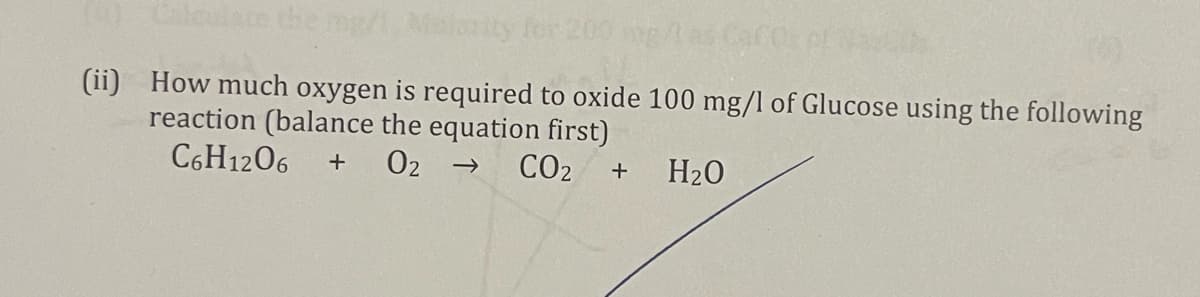
Transcribed Image Text:L0 Calculate the mg/
rity for 200 g
(ii) How much oxygen is required to oxide 100 mg/l of Glucose using the following
reaction (balance the equation first)
C6H1206
02 →
CO2
+
H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you


Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,


Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,