lat is the th CH,CH,OH + H,SO,→ CH,=CH, + H,0 OmL ethanol (MM: 46.07g/mol); ethylene (MM: 28.05); 30mL sulfuric acid 10 x 0.789g/mol = (7.89g x 1mol/46.07g) x (28.05g/1mol) = 4.80 g (L.R.) %3D 30 x 1.840g/mol = (55.2g x 1mol/98.08g) x (28.05/1mol) = 15.8 g %3D
lat is the th CH,CH,OH + H,SO,→ CH,=CH, + H,0 OmL ethanol (MM: 46.07g/mol); ethylene (MM: 28.05); 30mL sulfuric acid 10 x 0.789g/mol = (7.89g x 1mol/46.07g) x (28.05g/1mol) = 4.80 g (L.R.) %3D 30 x 1.840g/mol = (55.2g x 1mol/98.08g) x (28.05/1mol) = 15.8 g %3D
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.75P
Related questions
Question
TOPIC: Preparation of Ethylene
Expound and explain the given answer to the question.
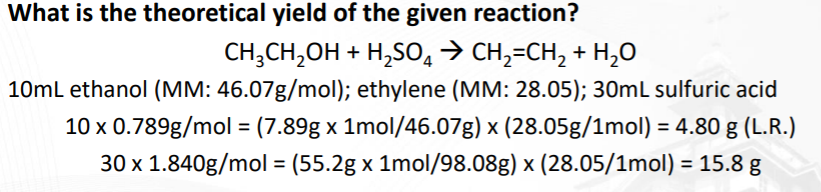
Transcribed Image Text:What is the theoretical yield of the given reaction?
CH,CH,OH + H,SO, > CH,=CH, + H,0
10ml ethanol (MM: 46.07g/mol); ethylene (MM: 28.05); 30mL sulfuric acid
10 x 0.789g/mol = (7.89g x 1mol/46.07g) x (28.05g/1mol) = 4.80 g (L.R.)
%3D
30 x 1.840g/mol = (55.2g x 1mol/98.08g) x (28.05/1mol) = 15.8 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning