Take the effective spring constant of a vibrating HCl molecule as 480 N/m. The mass of the hydrogen atom is 1.01 u. Assuming the chlorine atom is an atom of the isotope "CI, with atomic mass 35.0 u, find the energies of the ground state and of the 7th energy level, counting the ground state as the zeroth level.
Take the effective spring constant of a vibrating HCl molecule as 480 N/m. The mass of the hydrogen atom is 1.01 u. Assuming the chlorine atom is an atom of the isotope "CI, with atomic mass 35.0 u, find the energies of the ground state and of the 7th energy level, counting the ground state as the zeroth level.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter18: Raman Spectroscopy
Section: Chapter Questions
Problem 18.5QAP
Related questions
Question
Transcribed Image Text:< Q Hint
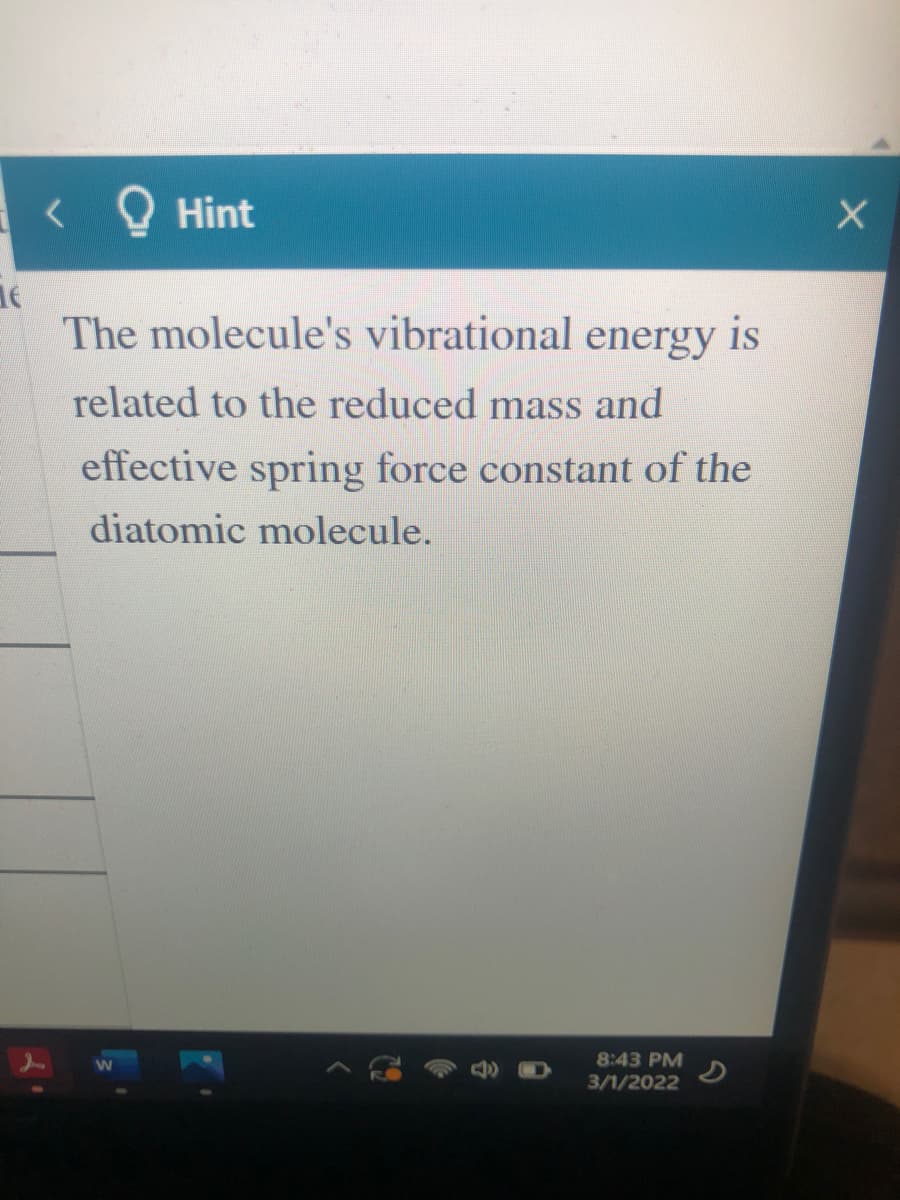
The molecule's vibrational energy is
related to the reduced mass and
effective spring force constant of the
diatomic molecule.
8:43 PM
3/1/2022
Transcribed Image Text:X Give Up
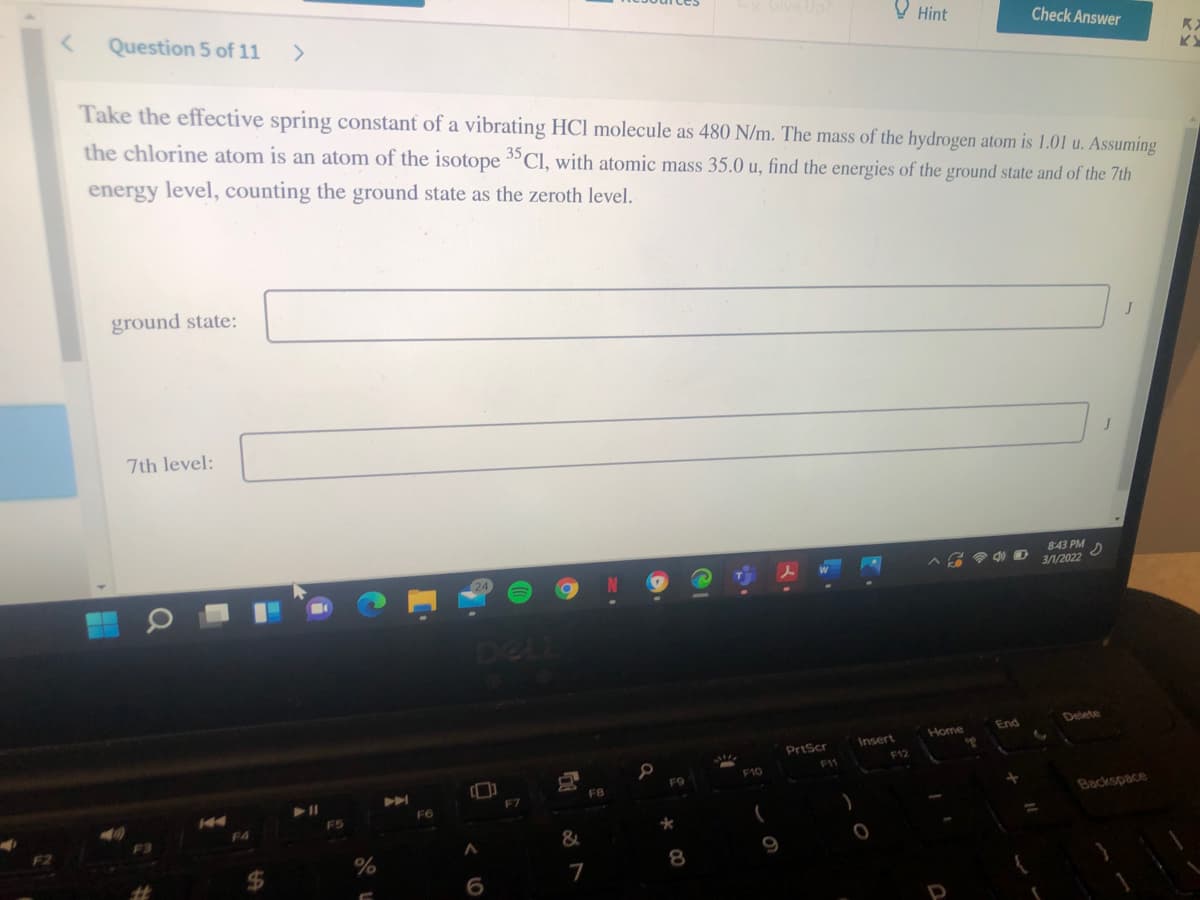
Question 5 of 11
O Hint
Check Answer
Take the effective spring constant of a vibrating HCl molecule as 480 N/m. The mass of the hydrogen atom is 1.01 u. Assuming
the chlorine atom is an atom of the isotope 3"Cl, with atomic mass 35.0 u, find the energies of the ground state and of the 7th
energy level, counting the ground state as the zeroth level.
ground state:
7th level:
J
8:43 PM
3/1/2022
Delete
End
Home
Insert
PriScr
F12
F11
F10
F9
FB
F7
F6
Backspace
F5
F4
%3D
%
8
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
