Lead (II) nitrate is reacted with sodium iodide. Calculate the number of Pb2+ ions required to form 0.99 g of precipitate. Write the balanced molecular, total ionic, and net ionic equations of the reaction. Atomic Mass: Pb: 207.2 g/mol N: 14.0067 g/mol O: 15.999 g/mol Na: 22.989769 g/mol I: 126.90447 g/mol Note: Use scientific notation with the format (n)e(x) where n = number and x = exponent (Example: 6.022e23 for 6.022 x 1023) Round your answer to 4 decimal places.
Lead (II) nitrate is reacted with sodium iodide. Calculate the number of Pb2+ ions required to form 0.99 g of precipitate. Write the balanced molecular, total ionic, and net ionic equations of the reaction. Atomic Mass: Pb: 207.2 g/mol N: 14.0067 g/mol O: 15.999 g/mol Na: 22.989769 g/mol I: 126.90447 g/mol Note: Use scientific notation with the format (n)e(x) where n = number and x = exponent (Example: 6.022e23 for 6.022 x 1023) Round your answer to 4 decimal places.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 14QAP: Aluminum ions react with carbonate ions to form an insoluble compound, aluminum carbonate. (a) Write...
Related questions
Question
Please show your complete solution.
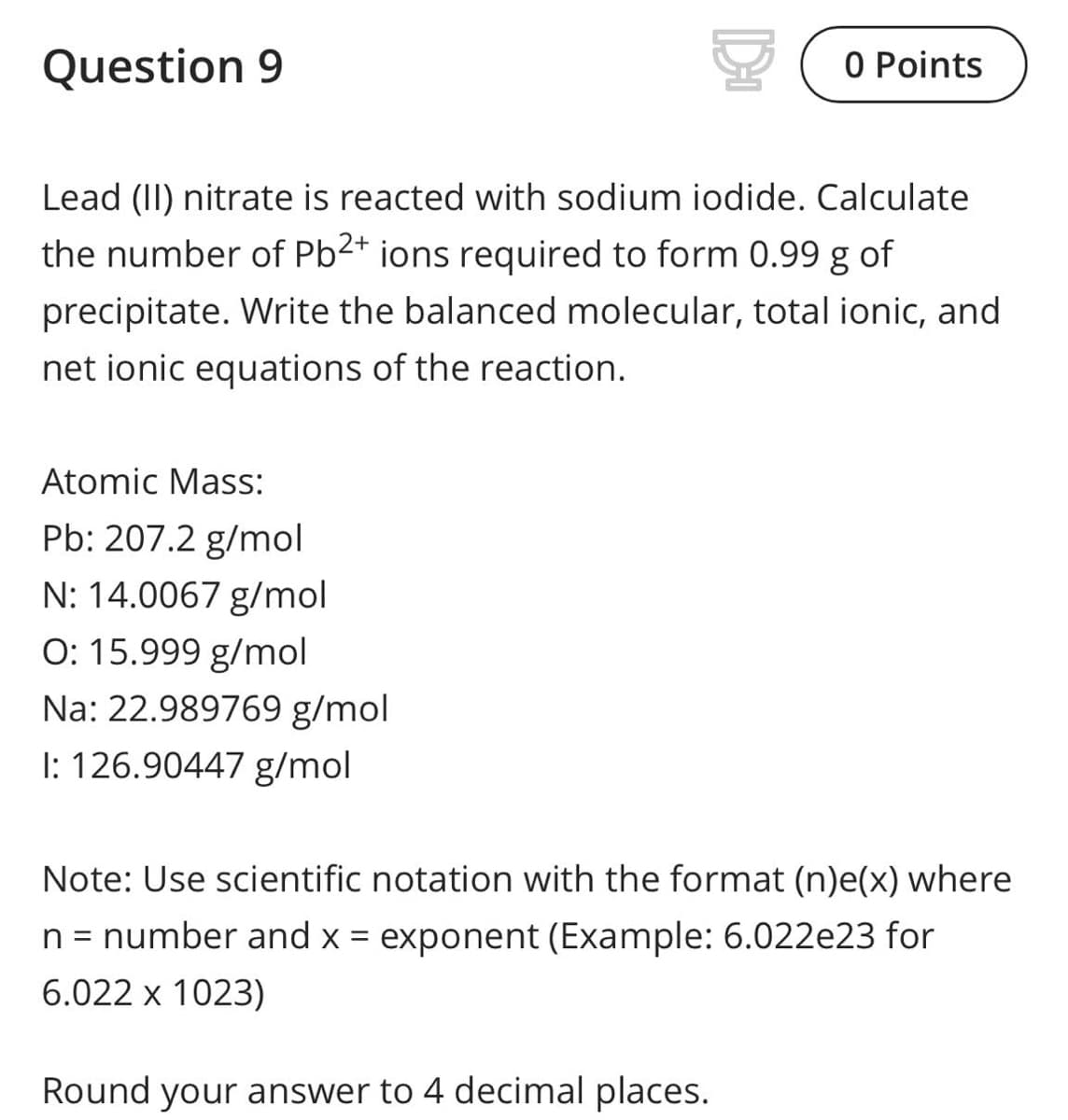
Transcribed Image Text:Question 9
O Points
Lead (II) nitrate is reacted with sodium iodide. Calculate
the number of Pb2+ ions required to form 0.99 g of
precipitate. Write the balanced molecular, total ionic, and
net ionic equations of the reaction.
Atomic Mass:
Pb: 207.2 g/mol
N: 14.0067 g/mol
O: 15.999 g/mol
Na: 22.989769 g/mol
I: 126.90447 g/mol
Note: Use scientific notation with the format (n)e(x) where
n = number and x = exponent (Example: 6.022e23 for
6.022 x 1023)
Round your answer to 4 decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning