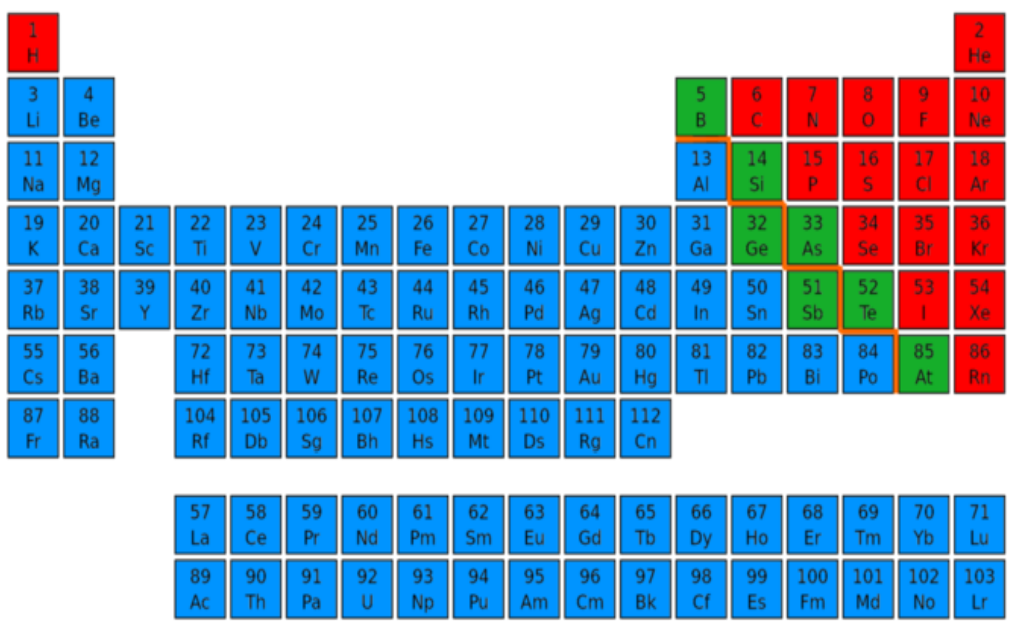
Learning Task No. 1: Using the Periodic Table of Elements, determine the ele- ments asked in each guide question. Write your answer in your notebook. Guide Questions: 1. Which elements are likely to lose electrons? 2. Which elements are likely to gain electrons? 3. Which type of elements are likely to have no electrical charge at all?
Learning Task No. 1: Using the Periodic Table of Elements, determine the ele- ments asked in each guide question. Write your answer in your notebook. Guide Questions: 1. Which elements are likely to lose electrons? 2. Which elements are likely to gain electrons? 3. Which type of elements are likely to have no electrical charge at all?
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter3: Electronic Structure And The Periodic Law
Section: Chapter Questions
Problem 3.21E
Related questions
Question
Transcribed Image Text:Learning Task No. 1: Using the Periodic Table of Elements, determine the ele-
ments asked in each guide question. Write your answer in your notebook.
Guide Questions:
1. Which elements are likely to lose electrons?
2. Which elements are likely to gain electrons?
3. Which type of elements are likely to have no electrical charge at all?
Transcribed Image Text:13
25
41
4
49| 50
51
72
81
83
14
104
107
Dy
90
92
93
95
97
99
100
101
102
103
Es
Em
146
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co