Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 4P
Related questions
Question
100%
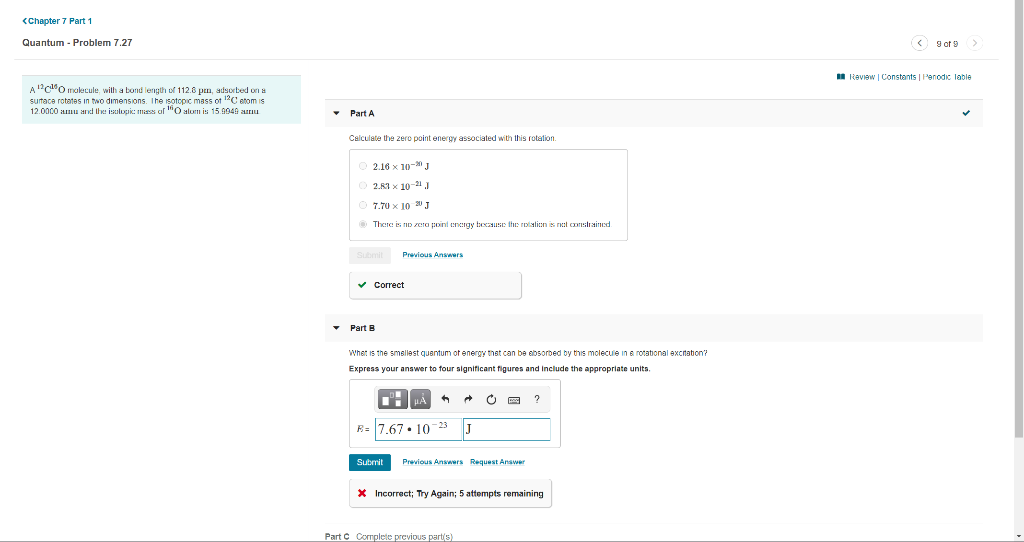
Transcribed Image Text:<Chapter 7 Part 1
Quantum - Problem 7.27
O 9 of 9>
I Review | Constants | Penodic lable
A"c"Omokecule, wilth a bond length of 112.6 pn, adsorbed on a
sutace rotates in two dimensions. The isotopic mass ot 12C atom is
12.0000 amu and the isalopic: mass al "O alom is 15.9949 au
• Part A
Calculate the zero point energy associated with this rotation.
O 2.16 x 10- J
2.R1 x 10-21 J
O 7.70 x 10 20 J
O There is nD zera point energy because the roialien is net coonslrained
Submit
Previous Answers
v Correct
• Part B
Whet is the smsllest quentum of energy thst can be ebsorbed by this molecule in a rotational excrtation?
Express your answer to four significant figures and include the appropriate units.
R= 7.67• 10-23
J
Submit
Previous Answers Request Answer
* Incorrect; Try Again; 5 attempts remaining
Part C Cormplete previous parts)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning