Limiting reactants ƏUPIRN Liquid octane (CH, (CH,) CH, will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). Suppose 18. g of octane Is mixed with 115. g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to 2 significant digits. x10 olb Ar O Chrome OS System 46m Sign-in has changed Account update required Explanation Check 2020 McGraw-Hill E OV 10:16 DELL
Limiting reactants ƏUPIRN Liquid octane (CH, (CH,) CH, will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). Suppose 18. g of octane Is mixed with 115. g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to 2 significant digits. x10 olb Ar O Chrome OS System 46m Sign-in has changed Account update required Explanation Check 2020 McGraw-Hill E OV 10:16 DELL
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 20A
Related questions
Question
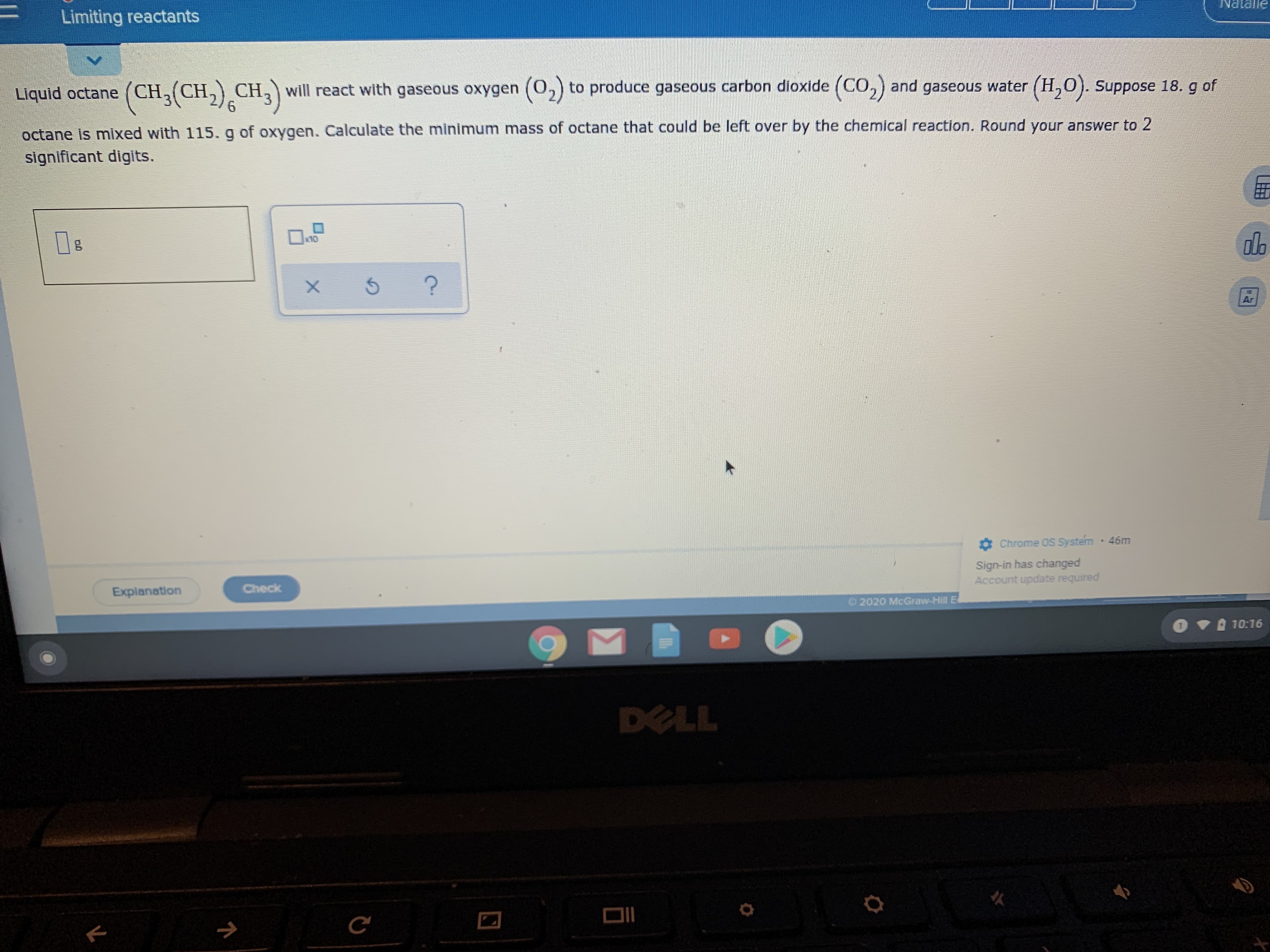
Transcribed Image Text:Limiting reactants
ƏUPIRN
Liquid octane (CH, (CH,) CH, will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). Suppose 18. g of
octane Is mixed with 115. g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to 2
significant digits.
x10
olb
Ar
O Chrome OS System 46m
Sign-in has changed
Account update required
Explanation
Check
2020 McGraw-Hill E
OV 10:16
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning