LIMITING REAGENTS T02/S22 Based on the balanced equation Molar Mass (g/mol) 2NBr3 + 3NaoH - N2 + 3NaBr + зновг NB13 253.72 calculate the number of of excess reagent units remaining when 104 NBR3 molecules and 147 NaOH 39.997 NAOH formula units react? N2 28.013 NaBr НOBr Avogadro's No. 6.022x1023 mol"1 102.800 96.911 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER BASIC STOICHIOMETRY T01/s11 Based on the balanced equation, Molar Mass (g/mol) 10Lİ + N2F4 - 4LİF + 2Lİ3N Li 6.941 calculate the molecules of N,Fa required when 56 formula units of LI3N are formed. N2F4 104.00 LIF 25.939 LizN Avogadro's No. 6.022x1023 mol1 34.830 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
LIMITING REAGENTS T02/S22 Based on the balanced equation Molar Mass (g/mol) 2NBr3 + 3NaoH - N2 + 3NaBr + зновг NB13 253.72 calculate the number of of excess reagent units remaining when 104 NBR3 molecules and 147 NaOH 39.997 NAOH formula units react? N2 28.013 NaBr НOBr Avogadro's No. 6.022x1023 mol"1 102.800 96.911 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER BASIC STOICHIOMETRY T01/s11 Based on the balanced equation, Molar Mass (g/mol) 10Lİ + N2F4 - 4LİF + 2Lİ3N Li 6.941 calculate the molecules of N,Fa required when 56 formula units of LI3N are formed. N2F4 104.00 LIF 25.939 LizN Avogadro's No. 6.022x1023 mol1 34.830 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 65E: Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is...
Related questions
Question
can you solve these 2 and just provide the answer I solved most of the work I just wanna make sure to get the answer right before i submit it
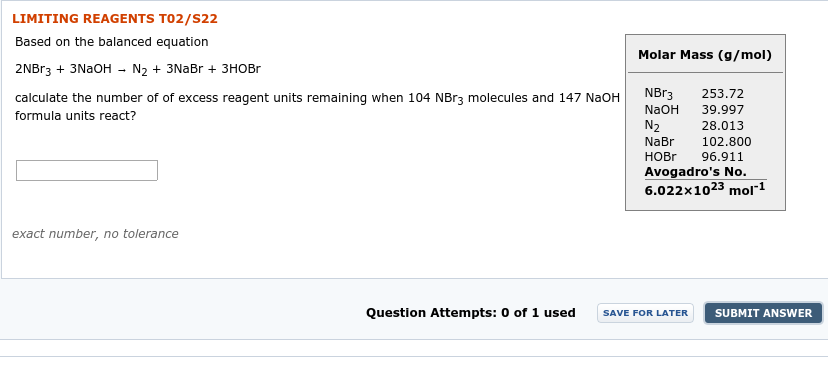
Transcribed Image Text:LIMITING REAGENTS T02/S22
Based on the balanced equation
Molar Mass (g/mol)
2NBr3 + 3NaoH - N2 + 3NaBr + зновг
NB13
253.72
calculate the number of of excess reagent units remaining when 104 NBR3 molecules and 147 NaOH
39.997
NAOH
formula units react?
N2
28.013
NaBr
НOBr
Avogadro's No.
6.022x1023 mol"1
102.800
96.911
exact number, no tolerance
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
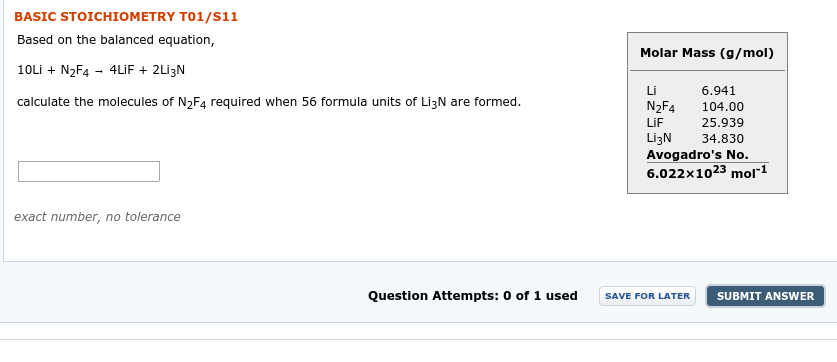
Transcribed Image Text:BASIC STOICHIOMETRY T01/s11
Based on the balanced equation,
Molar Mass (g/mol)
10Lİ + N2F4 - 4LİF + 2Lİ3N
Li
6.941
calculate the molecules of N,Fa required when 56 formula units of LI3N are formed.
N2F4
104.00
LIF
25.939
LizN
Avogadro's No.
6.022x1023 mol1
34.830
exact number, no tolerance
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co