Liquid bromine dissolves readily in aqueous NaOH: Br2(€) + 2 NaOH(aq) NaBr(aq) + NaOBr(aq) + H,O(€) Suppose 2.88 × 10-3 mol of Br2(e) is sealed in a glass cap- sule that is then immersed in a solution containing excess NaOH(aq). The capsule is broken, the mixture is stirred, and a measured 121.3 J of heat evolves. In a separate experiment, simply breaking an empty capsule and stirring the solution in the same way evolves 2.34 J of heat. Com- putc the heat cvolvcd as 1.00 mol Br,(l) dissolves in excess NaOH(aq).
Liquid bromine dissolves readily in aqueous NaOH: Br2(€) + 2 NaOH(aq) NaBr(aq) + NaOBr(aq) + H,O(€) Suppose 2.88 × 10-3 mol of Br2(e) is sealed in a glass cap- sule that is then immersed in a solution containing excess NaOH(aq). The capsule is broken, the mixture is stirred, and a measured 121.3 J of heat evolves. In a separate experiment, simply breaking an empty capsule and stirring the solution in the same way evolves 2.34 J of heat. Com- putc the heat cvolvcd as 1.00 mol Br,(l) dissolves in excess NaOH(aq).
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 165SCQ
Related questions
Question
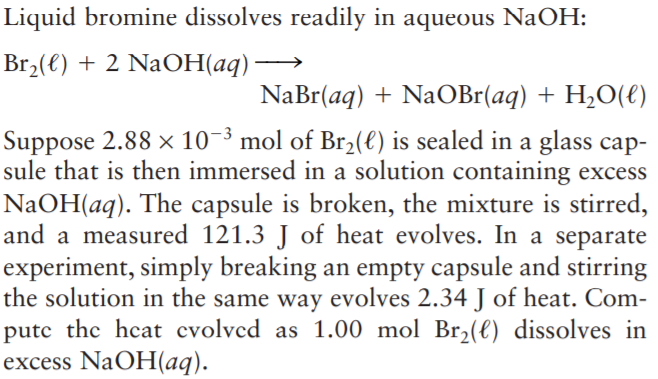
Transcribed Image Text:Liquid bromine dissolves readily in aqueous NaOH:
Br2(€) + 2 NaOH(aq)
NaBr(aq) + NaOBr(aq) + H,O(€)
Suppose 2.88 × 10-3 mol of Br2(e) is sealed in a glass cap-
sule that is then immersed in a solution containing excess
NaOH(aq). The capsule is broken, the mixture is stirred,
and a measured 121.3 J of heat evolves. In a separate
experiment, simply breaking an empty capsule and stirring
the solution in the same way evolves 2.34 J of heat. Com-
putc the heat cvolvcd as 1.00 mol Br,(l) dissolves in
excess NaOH(aq).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning