LizSO4 + H2O Type of Reaction: Balance Equation: 1. H2SO.+ LIO - Solution: 2. Al(NO.), + NH,OH AI(OH)3 + NH&NO3 Type of Reactlion. Balance Equation Solution. 3. Na + H20 NaOH + H2 Type of Reaction. Balance Equation Solution.
LizSO4 + H2O Type of Reaction: Balance Equation: 1. H2SO.+ LIO - Solution: 2. Al(NO.), + NH,OH AI(OH)3 + NH&NO3 Type of Reactlion. Balance Equation Solution. 3. Na + H20 NaOH + H2 Type of Reaction. Balance Equation Solution.
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.20QAP
Related questions
Question
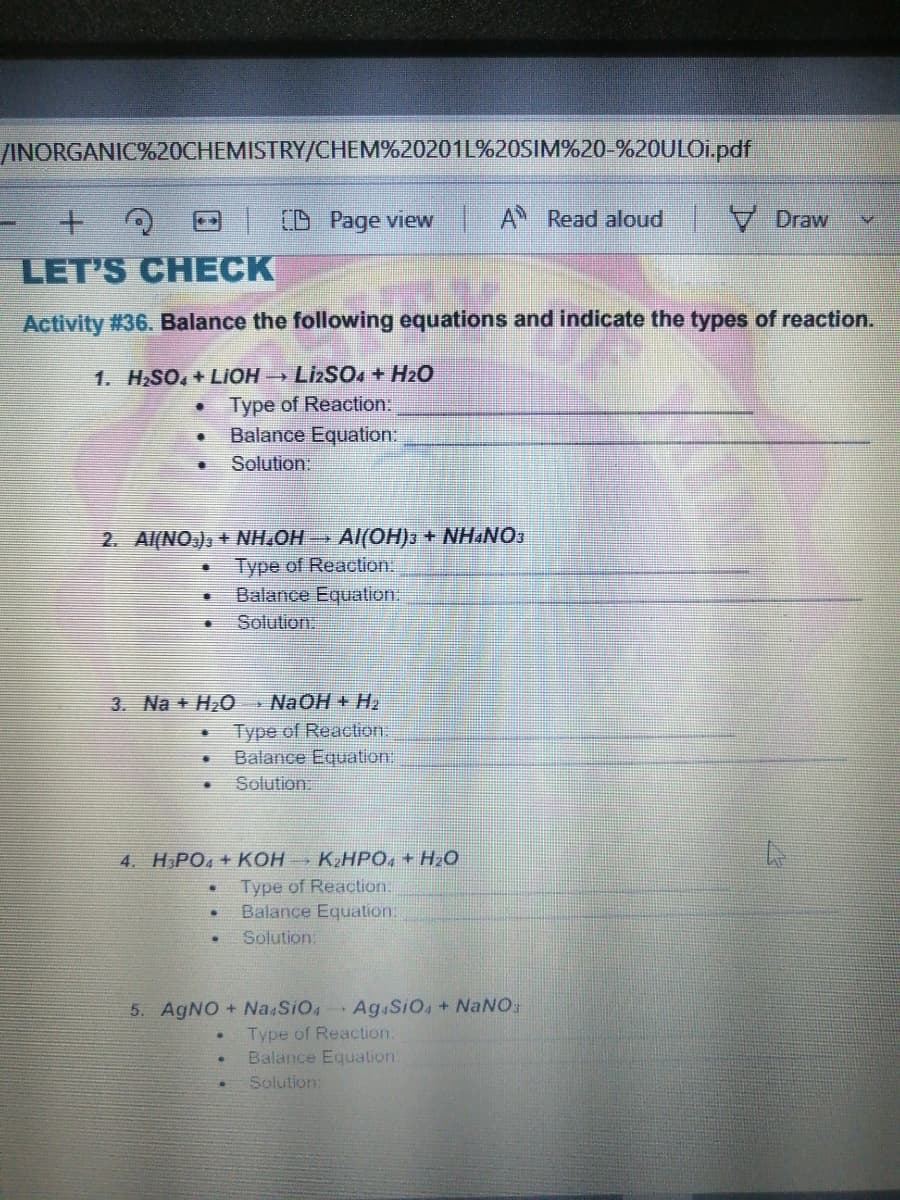
Transcribed Image Text:/INORGANIC%20CHEMISTRY/CHEM%20201L%20SIM%20-%20ULOI.pdf
(D Page view A Read aloud V Draw
LET'S CHECK
Activity #36. Balance the following equations and indicate the types of reaction.
1. HSO, + LIOH LIZSO + H2O
Type of Reaction:
Balance Equation:
Solution:
2. Al(NO.), + NH,OH
Al(OH)3 + NH&NOS
Type of Reaction:
Balance Equation:
Solution
3. Na + H20 NaOH + H,
Type of Reaction.
Balance Equation
Solution.
4. H,PO4 + KOH KZHPO, + H2O
Type of Reaction:
Balance Equation.
Solution:
5. AGNO + Na SiO4
Ag.SiO, + NaNO;
Type of Reaction.
Balance Equalion.
Solution:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
