How many grams of Ag,CO3 will precipitate when excess (NH4)½CO3 solution is added to 57.0 mL of 0.561 M AgNO3 solution? SS Visited 2A£NO3(aq) + (NH4)½CO3(aq) Ag,CO3(s) + 2NH4NO3(aq)
How many grams of Ag,CO3 will precipitate when excess (NH4)½CO3 solution is added to 57.0 mL of 0.561 M AgNO3 solution? SS Visited 2A£NO3(aq) + (NH4)½CO3(aq) Ag,CO3(s) + 2NH4NO3(aq)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 62QAP: Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker...
Related questions
Question
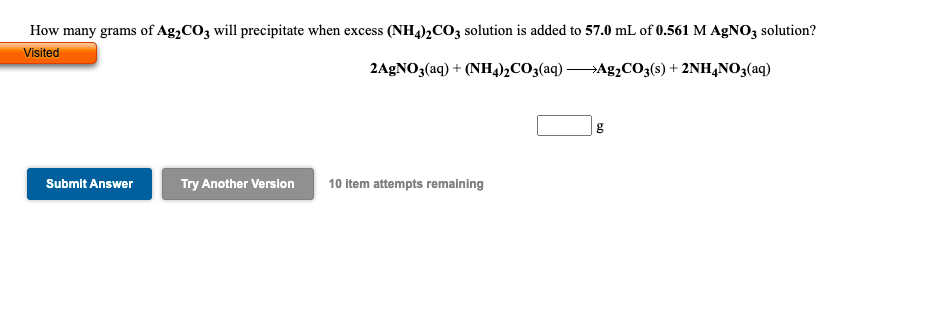
Transcribed Image Text:How many grams of Ag,CO3 will precipitate when excess (NH4)2CO3 solution is added to 57.0 mL of 0.561 M AgNO3 solution?
Visited
2AGNO3(aq) + (NH4)½CO3(aq) Ag¿CO3(s) + 2NH,N0;(aq)
g
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning